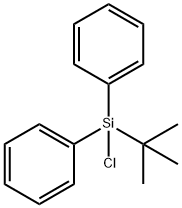
| CAS: | 58479-61-1 |
| MF: | C16H19ClSi |
| MW: | 274.86 |
| EINECS: | 261-282-0 |
| Product Categories: | Biochemistry;Monochlorosilanes;FINE Chemical & INTERMEDIATES;Nucleosides, Nucleotides & Related Reagents;Protecting Agents for Hydroxyl and Amino Groups;Protecting Agents, Phosphorylating Agents & Condensing Agents;Protection & Derivatization Reagents (for Synthesis);Reagents for Oligosaccharide Synthesis;Si (Classes of Silicon Compounds);Si-Cl Compounds;Silicon Compounds (for Synthesis);Synthetic Organic Chemistry;Blocking Agents;Phenyl Silanes;Protective Agents;Silylating Agents;bc0001 |
| Mol File: | 58479-61-1.mol |
 |
|
| tert-Butylchlorodiphenylsilane Chemical Properties |
| Boiling point | 90 °C0.01 mm Hg(lit.) |
| density | 1.057 g/mL at 25 °C(lit.) |
| refractive index | n20/D 1.568(lit.) |
| Fp | >230 °F |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | miscible in most organic solvents. |
| form | Liquid |
| color | Clear colorless to yellow or slightly brown |
| Specific Gravity | 1.074 |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 644023 |
| CAS DataBase Reference | 58479-61-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, chloro(1,1-dimethylethyl)diphenyl-(58479-61-1) |
| EPA Substance Registry System | Silane, chloro(1,1-dimethylethyl)diphenyl- (58479-61-1) |
| tert-Butylchlorodiphenylsilane Usage And Synthesis |
| Chemical Properties | Tert-Butylchlorodiphenylsilane is a colorless to pale brown oily liquid with pungent odor, may be used as silylating agent for derivatization of alcohols, ketones, carboxylic acids, amines, amides and mercaptanes selectively into functional groups in different sterical environments. |
| Physical properties | colorless liquid, bp 93–95°C/0.015 mmHg; n20 D 1.5680; d 1.057 g cm?3. |
| Uses | Several newmethods have been developed for using the reagent to protect primary and secondary alcohols as their TBDPS ethers. In the presence of ammonium nitrate or ammonium perchlorate, reaction between TBDPS-Cl and a primary alcohol, such as benzyl alcohol, in DMF provided excellent yields of the corresponding silyl ethers in just 15 min (eq 1).19 When silver nitrate was used as promoter, the reactions gave inferior yields under otherwise identical conditions.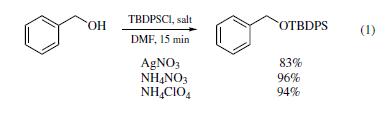
When TBDPS-Cl is used to react with hemiacetals, it converts hemiacetals into ring-opened silyl ether carbonyl compounds, instead of mixed acetals.Presumably, the sizable TBDPS group presents too much steric hindrance for the formation of the corresponding mixed silyl acetals. |
| Uses | tert-Butyldiphenylchlorosilane acts as a silylating reagent used to protect alcohols and in the preparation of silyl ethers. It plays a major role as a raw material and a precursor in organic synthesis and pharmaceuticals. It is also used for the synthesis of interphenylene phenyloxazoles which can be used for the treatment of circulatory disorders, angina and stroke. |
| Uses | tert-Butylchlorodiphenylsilane can be used to prepare 1-benzyloxy-3-(tert-butyldiphenylsilyloxy)propan-2-ol, a key intermediate for the synthesis of mono-O-protected pyrimidine acyclic nucleosides. |
| Preparation | a dry 1 L, three-necked round bottomed flask is equipped with a magnetic stirring bar, a 500mL equalizing dropping funnel fitted with a rubber septum, a reflux condenser, and nitrogen inlet tube. The flask is flushed with nitrogen, then charged with 127 g (0.5 mol) of diphenyldichlorosilane in 300mL of redistilled pentane. A solution of tbutyllithium in pentane (500 mL, 0.55 mol), is transferred under nitrogen pressure to the dropping funnel using a stainless steel, double-tip transfer needle. This solution is slowly added to the contents of the flask and when the addition is complete, the mixture is refluxed 30 h under nitrogen with stirring. The suspension is allowed to cool to rt, the precipitated lithium chloride is rapidly filtered through a pad of Celite, and the latter is washed with 200mL of pentane. The solvent is removed by evaporation, and the colorless residue is distilled through a short (10 cm), Vigreux column, to give 125–132 g of the colorless title compound. |
| Purification Methods | Purify it by repeated fractional distillaton. It is soluble in DMF and pentane [Hanessian & Lavalee Can J Chem 53 2975 1975, Robl et al. J Med Chem 34 2804 1991]. [Beilstein 4 IV 4076 for tert-butylchlorodimethylsilane.] |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock


 Japan
Japan








 Company information
Company information Advantage
Advantage


