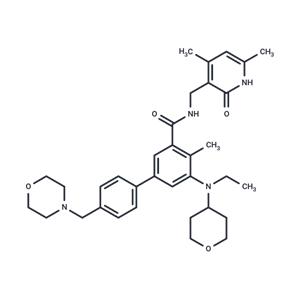
| Name | Tazemetostat |
| Description | Tazemetostat (EPZ6438) is a histone methyltransferase EZH2 inhibitor (IC50=11 nM) that is orally active, selective, and SAM-competitive. Tazemetostat exhibits antitumor activity and may be used for the treatment of epithelioid sarcoma/follicular lymphoma. |
| Cell Research | For the adherent cell line proliferation assays, plating densities for each cell line are determined based on growth curves (measured by ATP content) and density over a 7-d time course. On the day before compound treatment, cells are plated in either 96-well plates in triplicate (for the day 0–7 time course) or 6-well plates (for replating on day 7 for the remainder of the time course). On day 0, cells are either untreated, DMSO-treated, or treated with EPZ-6438 starting at 10 μM and decreasing in either threefold or fourfold dilutions. Plates are read on day 0, day 4, and day 7 using Cell Titer Glo, with compound/media being replenished on day 4. On day 7, the six-well plates are trypsinized, centrifuged, and resuspended in fresh media for counting by Vi-Cell. Cells from each treatment are replated at the original density in 96-well plates in triplicate. Cells are allowed to adhere to the plate overnight, and cells are treated as on day 0. On days 7, 11, and 14, plates are read using Cell Titer Glo, with compound/media being replenished on day 11. Averages of triplicates are used to plot proliferation over the time course, and calculate IC50 values. For cell cycle and apoptosis, G401 and RD cells are plated in 15-cm dishes in duplicate at a density of 1 × 106 cells per plate. Cells are incubated with EPZ-6438 at 1 μM, in a total of 25 mL, over a course of 14 d, with cells being split back to original plating density on day 4, 7, and 11. Cell cycle analysis and TUNEL assay are performed using a Guava flow cytometer, following the manufacturer's protocol.(Only for Reference) |
| Kinase Assay | Biochemical Methods: EPZ-6438 is incubated for 30 min with 40 μL per well of 5 nM PRC2 (final assay concentration in 50 μL is 4 nM ) in 1X assay buffer (20 mM Bicine [pH 7.6], 0.002% Tween-20, 0.005% Bovine Skin Gelatin and 0.5 mM DTT). 10 μL per well of substrate mix comprising assay buffer 3 H-SAM, unlabeled SAM, and peptide representing histone H3 residues 21-44 containing C-terminal biotin (appended to a C-terminal amide-capped lysine) are added to initiate the reaction (both substrates are present in the final reaction mixture at their respective Km values, an assay format referred to as ‘‘balanced conditions''. The final concentrations of substrates and methylation state of the substrate peptide are indicated for each enzyme Reactions are incubated for 90 min at room temperature and quenched with 10 μL per well of 600 μM unlabeled SAM, Then transferred to a 384-well flashplate and washed after 30 min. |
| In vitro | METHODS: Synovial sarcoma cells Fuji and HS-SY-II were treated with Tazemetostat (0.039-20 µmol/L) for 14 days, and cell viability was measured using the CellTiter-Glo Luminescent Cell Viability Assay.
RESULTS: Concentration-dependent reduction of cell proliferation was observed in Tazemetostat-treated Fuji and HS-SY-II cells, with IC50 values of 0.15 µmol/L and 0.52 µmol/L, respectively. [1]
METHODS: Human renal cancer cells G401 and human malignant embryonic rhabdomyosarcoma cells RD were treated with Tazemetostat (0.006-1100 nM) for 4 days, and the expression levels of the target proteins were detected by Western Blot.
RESULTS: Tazemetostat treatment resulted in a concentration-dependent decrease in overall H3K27Me3 levels. [2]
Translated with DeepL.com (free version) |
| In vivo | METHODS: To assay antitumor activity in vivo, Tazemetostat (125-500 mg/kg, 0.5% NaCMC plus 0.1% Tween 80 in water) was administered orally to SCID mice harboring human renal carcinoma tumor G401 twice a day for twenty-eight days.
RESULTS: Tazemetostat caused complete and sustained regression of SMARCB1 mutant MRT xenografts. [1]
METHODS: To investigate the role in subarachnoid hemorrhage (SAH)-induced neuroinflammation, Tazemetostat (1-9 mg/kg) was administered intraperitoneally to a rat model of subarachnoid hemorrhage induced by endovascular perforation.
RESULTS: Inhibition of EZH2 by Tazemetostat attenuated neuroinflammation after SAH in rats via the H3K27me3/SOCS3/TRAF6/NF-κB signaling pathway. [3] |
| Storage | store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | 10% DMSO+40% PEG300+5% Tween 80+45% Saline : 3.3 mg/mL (5.76 mM), Solution.
DMSO : 32.19 mg/mL (56.2 mM), Sonication and heating are recommended.
H2O : < 1 mg/mL (insoluble or slightly soluble)
Ethanol : < 1 mg/mL (insoluble or slightly soluble)
|
| Keywords | Tazemetostat | PRC2 | Inhibitor | inhibit | HistoneMethyltransferase | Histone Methyltransferase | EZH2 | EZH1 | EPZ-6438 | EPZ 6438 | epigenetic | E7438 | E 7438 | cancer |
| Inhibitors Related | Stavudine | Cysteamine hydrochloride | Sodium 4-phenylbutyrate | Metronidazole | Citric Acid Triammonium | Tributyrin | L-Methionine | Sodium citrate | Cystamine dihydrochloride | L-Ascorbic acid sodium salt | Alginic acid | Dextran sulfate sodium salt (MW 5000) |
| Related Compound Libraries | Highly Selective Inhibitor Library | Bioactive Compound Library | Membrane Protein-targeted Compound Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Inhibitor Library | Anti-Cancer Approved Drug Library | FDA-Approved Drug Library | Anti-Aging Compound Library | Bioactive Compounds Library Max | Anti-Cancer Drug Library | Anti-Cancer Active Compound Library |

 United States
United States