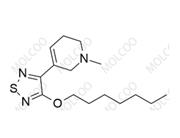
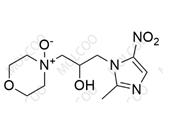
Suplatast tosilate Impurity 8

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Advantages:
This product has high purity, which can accurately meet the strict requirements for impurity reference substances in suplatast tosilate drug quality control and related research. It has good chemical stability and can maintain stable physical and chemical properties for a long time under standardized storage conditions, providing a strong guarantee for the repeatability and accuracy of experiments. Strict quality control is implemented during the production process, with small differences between batches, which can effectively reduce experimental errors and provide a reliable material basis for scientific research and production work.
Product Applications:
It is mainly used in the quality control field of suplatast tosilate, serving as an impurity reference substance to detect the content of this impurity in suplatast tosilate raw materials and various preparations, ensuring that the drug quality meets relevant standards. Moreover, in the research and development process of suplatast tosilate, it can be used to explore the formation mechanism of this impurity and its impact on drug efficacy and safety, providing important data references for optimizing production processes and improving drug quality.
Background Description:
Suplatast tosilate is a leukotriene synthesis inhibitor, mainly used in the treatment of allergic diseases such as bronchial asthma and allergic rhinitis. It reduces inflammatory reactions and allergic symptoms by inhibiting the synthesis of leukotrienes. During its synthesis, storage and preparation processes, various impurities may be generated due to factors such as incomplete reactions, raw material impurities or degradation, and Suplatast tosilate Impurity 8 is one of them. If the content of these impurities exceeds the standard, it may affect the stability and efficacy of the drug, and even bring potential safety risks. Therefore, accurate detection and strict control of them are of great significance.
Research Status:
At present, research on Suplatast tosilate Impurity 8 mainly focuses on the development and optimization of detection methods, such as high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS), to improve the sensitivity and accuracy of detection. At the same time, studies on the source, stability of this impurity and its interaction with the main component of suplatast tosilate are also gradually being carried out, providing a scientific basis for improving the quality standards and safety evaluation system of suplatast tosilate
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com






 China
China