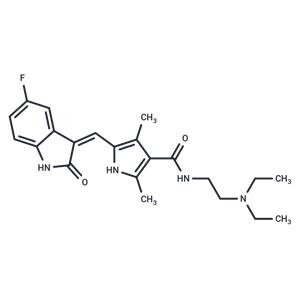
| Name | Sunitinib |
| Description | Sunitinib (SU 11248) is a multi-targeted receptor tyrosine kinase (RTK) inhibitor that inhibits VEGFR2 and PDGFRβ (IC50=80/2 nM). It exhibits antitumor activity and is used for treating kidney cancer and gastrointestinal tumors. |
| Cell Research | Cells are starved overnight in medium containing 0.1% FBS prior to addition of Sunitinib and FL (50 ng/mL; FLT3-WT cells only). Proliferation is measured after 48 hours of culture using the Alamar Blue assay or trypan blue cell viability assays. Apoptosis is measured 24 hours after Sunitinib addition by Western blotting to detect cleavage of poly (ADP-ribose) polymerase (PARP) or levels of caspase-3. (Only for Reference) |
| Kinase Assay | Biochemical Tyrosine Kinase Assays: IC50 values for Sunitinib against VEGFR2 (Flk-1) and PDGFRβ are determined using glutathione S-transferase fusion proteins containing the complete cytoplasmic domain of the RTK. Biochemical tyrosine kinase assays to quantitate the trans-phosphorylation activity of VEGFR2 (Flk-1) and PDGFRβ are performed in 96-well microtiter plates precoated (20 μg/well in PBS; incubated overnight at 4 °C) with the peptide substrate poly-Glu,Tyr (4:1). Excess protein binding sites are blocked with the addition of 1-5% (w/v) BSA in PBS. Purified GST-fusion proteins are produced in baculovirus-infected insect cells. GST-VEGFR2 and GST-PDGFRβ are then added to the microtiter wells in 2 × concentration kinase dilution buffer consisting of 100 mM HEPES, 50 mM NaCl, 40 μM NaVO4, and 0.02% (w/v) BSA. The final enzyme concentration for GST-VEGFR2 or GST-PDGFRβ is 50 ng/mL. Twenty-five μL of diluted Sunitinib are subsequently added to each reaction well to produce a range of inhibitor concentrations appropriate for each enzyme. The kinase reaction is initiated by the addition of different concentrations of ATP in a solution of MnCl2 so that the final ATP concentrations spanned the Km for the enzyme, and the final concentration of MnCl2 is 10 mM. The plates are incubated for 5-15 minutes at room temperature before stopping the reaction with the addition of EDTA. The plates are then washed three times with TBST. Rabbit polyclonal antiphosphotyrosine antisera are added to the wells at a 1:10,000 dilution in TBST containing 0.5% (w/v) BSA, 0.025% (w/v) nonfat dry milk, and 100 μM NaVO4 and incubated for 1 hour at 37 °C. The plates are then washed three times with TBST, followed by the addition of goat antirabbit antisera conjugated with horseradish peroxidase (1:10,000 dilution in TBST). The plates are incubated for 1 hour at 37 °C and then washed three times with TBST.The amount of phosphotyrosine in each well is quantitated after the addition of 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate] as substrate. |
| In vitro | METHODS: FLT3-ITD cell line MV4;11 and FLT3-WT cell line OC1-AML5 were treated with Sunitinib (0.001-10 µM) for 48 h, and cell viability was assayed using Alamar blue assay.
RESULTS: Sunitinib significantly inhibited the proliferation of MV4;11 and OC1-AML5 cells in a dose-dependent manner, with IC50s of 8 nM and 14 nM, respectively. [1]
METHODS: GIST-T1 gastrointestinal mesenchymal stromal tumor cells were treated with Sunitinib (10-40 nM) for 48 h, and the expression levels of target proteins were detected by Western Blot.
RESULTS: Sunitinib inhibited the autophosphorylation of c-KIT in a dose-dependent manner, and effectively blocked the phosphorylation of the downstream effectors of c-KIT, Akt and ERK, without affecting the phosphorylated forms of STAT3 and STAT5. [2] |
| In vivo | METHODS: To assay antitumor activity in vivo, Sunitinib (1-40 mg/kg in a citrate-buffered solution (pH 3.5)) was administered by gavage to athymic nu/nu mice harboring human leukemia tumors MV4;11 once a day until the tumors regressed.
RESULTS: Sunitinib demonstrated dose-dependent efficacy when administered at 4 0 and 20 mg/kg/d and regressed established large subcutaneous tumors. [1]
METHODS: To assay antitumor activity in vivo, Sunitinib (40-80 mg/kg in CMC) was administered by gavage to athymic nu/nu mice injected with breast cancer cells MDA-MB-435/HAL once a day for twenty-one days.
RESULTS: Sunitinib treatment of hormonal mice resulted in a significant reduction in serum PYD levels, confirming the in vivo inhibitory effect of osteolysis. Using BLI, Sunitinib showed 64% inhibition of bone tumor growth at 40 mg/kg/day for 21 days of treatment and 89% inhibition at 80 mg/kg/day. [3] |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 25 mg/mL (62.74 mM)
10% DMSO+40% PEG300+5% Tween 80+45% Saline : 1.25 mg/mL (3.14 mM), Please add co-solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately.
H2O : < 1 mg/mL (insoluble or slightly soluble)
Ethanol : < 1 mg/mL (insoluble or slightly soluble)
|
| Keywords | Sunitinib | PDGFR | Inositol requiring enzyme 1 | IRE1 | Platelet-derived growth factor receptor | Autophagy | Mitophagy | Vascular endothelial growth factor receptor | inhibit | Inhibitor | SU11248 | VEGFR | SU-11248 | Apoptosis | Mitochondrial Autophagy |
| Inhibitors Related | Stavudine | Sodium 4-phenylbutyrate | Hydroxychloroquine | Guanidine hydrochloride | Tributyrin | Paeonol | Naringin |
| Related Compound Libraries | Membrane Protein-targeted Compound Library | Tyrosine Kinase Inhibitor Library | Anti-Cancer Clinical Compound Library | Inhibitor Library | Anti-Cancer Approved Drug Library | FDA-Approved Kinase Inhibitor Library | FDA-Approved Drug Library | Anti-Aging Compound Library | Bioactive Compounds Library Max | Anti-Cancer Active Compound Library |

 United States
United States