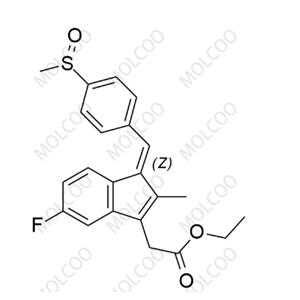
Sulindac Impurity 12;68299-97-8
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Number: S037012
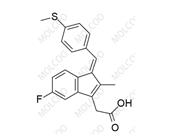
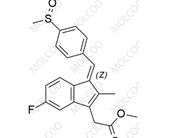
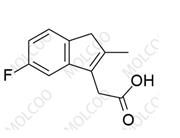
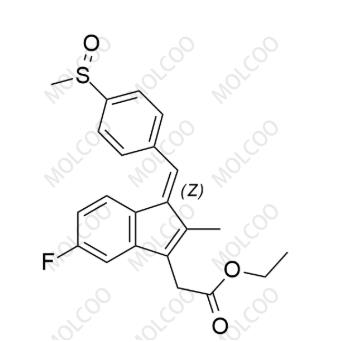
English Name: Sulindac Impurity 12
English Alias: (Z)-ethyl 2-(5-fluoro-2-methyl-1-(4-(methylsulfinyl)benzylidene)-1H-inden-3-yl)acetate
CAS Number: 68299-97-8
Molecular Formula: C₂₂H₂₁FO₃S
Molecular Weight: 384.46
As an impurity of Sulindac, this compound has the following advantages:
Well-defined with distinct stereochemical features: Contains an indene core, (Z)-benzylidene double bond, 5-fluoro substitution, methylsulfinyl (-S(O)-CH₃), and ethyl acetate side chain. Unlike sulindac (a carboxylic acid), its ethyl ester group and (Z)-configuration enable clear differentiation via reversed-phase HPLC/GC-MS as a specific impurity marker;
High stability and traceability: Rigid indene structure and sulfinyl stability ensure stability under neutral to weakly alkaline conditions. As an intermediate from incomplete ethyl ester hydrolysis in sulindac synthesis, it directly reflects ester hydrolysis efficiency, improving process tracing accuracy;
High detection sensitivity: UV absorption (270-290nm) from (Z)-alkene-benzene-indene conjugation, combined with characteristic mass response (m/z 385 [M+H]⁺), enables trace analysis (ppb level) via LC-MS or GC-MS, compatible with indene-based NSAID ethyl ester impurity systems.
Pharmaceutical quality control: Used as an impurity reference standard to quantify Sulindac Impurity 12 in APIs, ensuring residual ethyl ester intermediates meet quality standards post-ester hydrolysis/indene synthesis;
Synthesis optimization: Optimizing ethyl ester hydrolysis (reaction time, catalyst type) by monitoring impurity levels to enhance carboxylic acid formation efficiency;
Intermediate purity assessment: Evaluating purity of key (Z)-ethyl ester intermediates in sulindac synthesis to support specificity of downstream hydrolysis/oxidation.
Sulindac synthesis often uses ethyl acetate derivatives as intermediates, with target carboxylic acid formed via (Z)-double bond retention and ester hydrolysis. Incomplete ethyl ester hydrolysis may generate unhydrolyzed ethyl acetate derivatives like Sulindac Impurity 12. With lower pharmacological activity due to unhydrolyzed ester, its residue risks reducing sulindac purity, making control critical for quality assurance.
Current research focuses on:
Analytical method validation: Developing RP-HPLC assays with C18 columns for baseline separation, achieving 0.1 ppm quantitation limits via UV detection (280nm);
Hydrolysis kinetics: Studying impurity formation under varying hydrolysis systems to clarify ethyl ester-to-carboxylic acid conversion mechanisms;
Process refinement: Controlling impurity levels below 0.05% via optimized hydrolysis parameters to enhance API purity;
Structural confirmation: Using ¹H/¹³C-NMR to verify (Z)-configuration and ethyl ester position, distinguishing from sulindac for authoritative impurity identification.
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com





 China
China