| Chemical Properties | white to cream powder |
| Originator | Gantrisin,Roche,US,1949 |
| Uses | Sulfisoxazole is a sulfonamide based antibacterial that exhibits activity against wide spectrum of gram-negative and gram-positive bacterium. |
| Definition | ChEBI: A sulfonamide antibacterial with an oxazole substituent. It has antibiotic activity against a wide range of gram-negative and gram-positive organisms. |
| Manufacturing Process | 112 parts of 3,4-dimethyl-5-amino-isoxazole were dissolved in a mixture of 100 volume parts of pyridine and 200 volume parts of acetone. The mixture is cooled with cold water and 240 parts p-acetamino-benzene sulfonic acid chloride are added in small portions under stirring at temperatures of below 30°C. The mixture is left standing overnight at 20° to 30°C and then the 5- acetamino-benzene-sulfonylamino-3,4-dimethyl-isoxazole is precipitated by the addition of water. Recrystallized from acetic acid or alcohol it forms small prisms of the melting point 210°C.
100 parts of the 5-acetamino-benzene-sulfonyl-amino-3,4-dimethyl-isoxazole are boiled under reflux with 500 volume parts 15 to 20% aqueous hydrochloric acid for 30 to 45 minutes until all is dissolved. 500 parts crystallized sodium acetate are added and the liquid left cooling for crystallization. The sulfanilamido-3,4-dimethyl-isoxazole is sucked off, washed with water and dried. In the pure state it forms white prisms with the melting point of 193°C. |
| Brand name | Gantrisin (Roche). |
| Therapeutic Function | Antibacterial |
| Antimicrobial activity | Like all examined sulfanilamides, this drug is effective in treating infections caused by streptococci, gonococci, pneumococci, staphylococci, and also colon bacillus. However, about 90% of it binds with proteins in the plasma after oral administration, and it dif�fuses mostly to tissues and tissue fluids, which makes it the drug of choice for many sys�temic infections. Synonyms of this drug are gantrisin, fultrxin, sulfazin, sulfolar, and others. |
| General Description | Sulfisoxazole’s plasmahalf-life is 6 hours. This compound is a white, odorless,slightly bitter, crystalline powder. Its pKa is 5.0. At pH 6,this sulfonamide has a water solubility of 350 mg in100 mL, and its acetyl derivative has a solubility of 110 mgin 100 mL of water.Sulfisoxazole possesses the action and the uses of othersulfonamides and is used for infections involving sulfonamide-sensitive bacteria. It is claimed to be effective in thetreatment of Gram-negative urinary infections. |
| General Description | Odorless white to yellowish crystalline powder. Slightly bitter taste. Acid to litmus. |
| Air & Water Reactions | May be sensitive to prolonged exposure to air and light. Sensitive to heat. Slightly soluble in water. |
| Fire Hazard | Flash point data for Sulfisoxazole are not available; however, Sulfisoxazole is probably combustible. |
| Pharmaceutical Applications | 3,4-Dimethyl-5-sulfanilamidoisoxazole. It is highly soluble, even in acid urine. The spectrum and potency are typical of the group. It is well absorbed, achieving a concentration of around 20 mg/L 3–4 h after a 2 g oral dose.
Side effects are those common to other sulfonamides. It is less prone than some other members of the group to cause renal problems. Its principal use is in urinary tract infection, and is present in some ophthalmic preparations. |
| Biological Activity | A selective ET A endothelin receptor antagonist (IC 50 values are 600 and 2200 nM for ET A and ET B receptors respectively). |
| Safety Profile | Mildly toxic by ingestion. An experimental teratogen. Questionable carcinogen. Mutation data reported. When heated to decomposition it emits very toxic fumes of SOx and NOx. |
| Synthesis | Sulfisoxazole, N1 -(3,4-dimethyl-5-isoxazolyl)sulfanilamide (33.1.19), is synthesized by reacting 4-acetylaminobenzenesulfonyl chloride with 5-amino-3, 4-dimethylisoxazol (33.1.17), which is in turn synthesized by heterocyclization of 2-methy�lacetylacetonitrile with hydroxylamine, and subsequent acidic hydrolysis (hydrochloric acid) of the protective acetyl group in the resulting product (33.1.18). 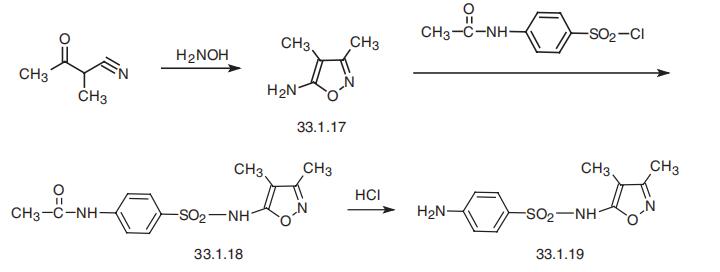
|