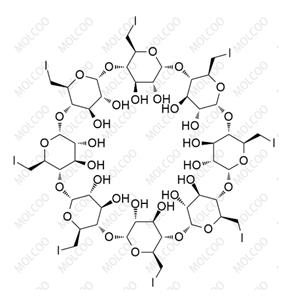
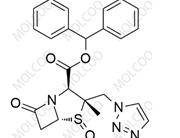
Sugammadex sodium Impurity
We can provide a full range of impurity reference/standard products required for drug development. Most of the impurities are synthesized through the process, there are also many items of impurities can not be obtained by synthetic means, need to be obtained through raw materials, intermediates, crude products or side reactions contained in the trace target compounds, Hubei Moke has a professional impurity preparation and separation technology team, equipped with professional SFC preparation and separation equipment. It can carry out efficient and accurate separation of impurities for complex projects, and solve the problem of impurity preparation for customers.

Sugammadex Sodium Impurity Reference Substances
Sugammadex sodium impurity reference substances are crucial reference materials in pharmaceutical research and development (R&D) and production processes. We offer a variety of sugammadex sodium impurity reference substances, including but not limited to impurities such as Org198786-1, Org246653-1, Org284426-1, Org199425-1, Org48302, Org199129-1, and Org49095. These impurity reference substances have undergone rigorous quality control and possess high purity (≥99%) and reliable structural characterization.
We provide Certificates of Analysis (COA), purity test reports, and conventional structural characterization data, such as 1H-NMR (Proton Nuclear Magnetic Resonance) and LCMS (Liquid Chromatography-Mass Spectrometry), along with the impurity reference substances. Additionally, customers can select additional testing services such as high-resolution mass spectrometry, infrared spectroscopy, ultraviolet spectroscopy, 13C-NMR (Carbon Nuclear Magnetic Resonance), and two-dimensional NMR based on their specific needs.
Our sugammadex sodium impurity reference substances are suitable for various stages of pharmaceutical R&D, quality control, and drug registration, serving as important tools to ensure the safety and effectiveness of pharmaceutical products.

 China
China