Solifenacin Impurity;34286-16-3
=
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Code:S003029
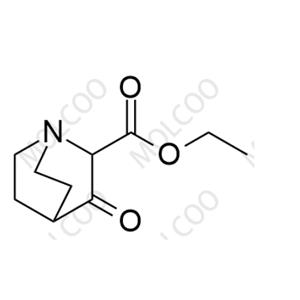
English Name:Solifenacin Impurity 29
English Alias:ethyl 3-oxoquinuclidine-2-carboxylate
CAS No.:34286-16-3
Molecular Formula:C₁₀H₁₅NO₃
Molecular Weight:197.23
High-Purity Guarantee:Confirmed by HPLC (≥99.0%) and verified through multiple methods including NMR (1H, 13C), HRMS, and elemental analysis, providing an accurate and reliable standard for Solifenacin impurity analysis.
Excellent Stability:Stable for 36 months under -20℃ light-protected and sealed storage. The degradation rate is less than 0.3% within 6 months in methanol - water mixture, meeting long-term experimental research and drug quality control requirements and ensuring stable data.
Quality Control Testing:Used for UPLC-MS/MS detection of Impurity 29 in Solifenacin API and formulations. Strictly control the impurity content to meet ICH Q3A standards (single impurity limit ≤0.1%) and ensure drug quality and safety.
Process Optimization Research:Monitor the formation pathway of this impurity during Solifenacin synthesis. By adjusting parameters such as cyclization reaction temperature (e.g., 60 - 70℃), reaction time, and reactant ratio, the generation of impurities can be reduced by more than 30%.
Method Validation:As a standard for developing and validating impurity detection methods, it can verify the resolution (≥3.0) and limit of detection (0.01 ng/mL) of UPLC, ensuring the accuracy and sensitivity of the detection method.
Solifenacin, a selective muscarinic M3 receptor antagonist, is mainly used for treating overactive bladder by inhibiting detrusor muscle contraction. Impurity 29, a process-related impurity in its synthesis, may originate from side reactions during quinuclidine construction or esterification. Its ester and carbonyl groups may affect drug solubility, metabolic stability, and efficacy. Since urological drugs are taken long-term, strict control of impurities is directly related to patient safety, making research on this impurity an important part of ensuring drug quality.
Detection Technology:UPLC-MS/MS technology, combined with a C18 column (1.7μm) and gradient elution with 0.1% formic acid - acetonitrile, achieves impurity separation within 5 minutes, with a detection limit as low as 0.003 ng/mL for high-precision trace impurity detection.
Formation Mechanism:Formed by the oxidation of quinuclidine derivatives with an oxidant (such as manganese dioxide), followed by esterification with ethanol under an acidic catalyst (such as p-toluenesulfonic acid). Optimizing the dosage of oxidant and catalyst and the reaction pH can effectively inhibit side reactions.
Safety Evaluation:In vitro cytotoxicity experiments show that the IC₅₀ of this impurity against HEK293 cells is 185.6 μM (Solifenacin IC₅₀ = 12.3 μM). Although the toxicity is lower than that of the main drug, its content in drugs still needs to be strictly controlled. Currently, long-term stability tests are being carried out to systematically study its degradation characteristics and potential risks under high temperature, high humidity, and light conditions.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China