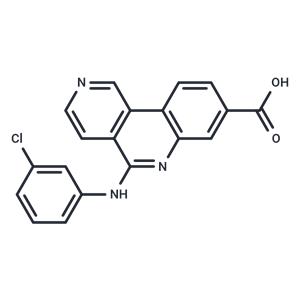
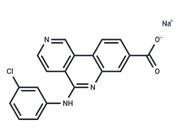
| Name | Silmitasertib |
| Description | Silmitasertib (CX-4945) is a potent, orally bioavailable inhibitor of casein kinase 2 (CK2; Ki: 0.38 nM). |
| Cell Research | Various cell lines were seeded at a density of 3000 cells per well 24 h prior to treatment, in appropriate media, and then treated with indicated concentrations of CK2 inhibitors. Suspension cells were seeded and treated on the same day. Following 4 days of incubation, 20 μL of Alamar Blue (10% of volume/well) was added and the cells were further incubated at 37 °C for 4-5 h. Fluorescence with excitation wavelength at 530-560 nm and emission wavelength at 590 nm was measured [3]. |
| Kinase Assay | The 238 kinase selectivity panel was conducted using the Kinase Profiler service, which utilizes a radiometric filter-binding assay. The percent inhibition of each kinase was estimated using 0.5 μmol/L CX-4945 at ATP concentrations equivalent to the Km value for ATP for each respective human recombinant kinase. The determination of IC50 values was done at ATP concentrations equivalent to the Km for ATP for each kinase using 9 concentrations of CX-4945 over a range of 0.0001 to 1 μmol/L. The Ki value (inhibition constant) for CX-4945 against recombinant CK2 was determined by graphing the IC50 values of CX-4945 determined in the presence of various concentrations of ATP against the concentration of ATP. The Ki value is equivalent to the Y-intercept according to the Cheng–Prussoff equation (Ki = IC50/(1+[ATP]/Km), where Ki is the inhibition constant and Km is the Michaelis constant [1]. |
| Animal Research | Xenografts were initiated by subcutaneous injection of BxPC-3 cells into the right hind flank region of each mouse or BT-474 cells were injected into the mammary fat pad of mice implanted with estrogen pellets. When tumors reached a designated volume of 150-200 mm^3, animals were randomized and divided into groups of 9 to 10 mice per group. CX-4945 was administered by oral gavage twice daily at 25 or 75 mg/kg for 31 and 35 consecutive days for the BT-474 and BxPC-3 models, respectively. Tumor volumes and body weights were measured twice weekly. The length and width of the tumor were measured with calipers and the volume calculated using the following formula: tumor volume = (length × width^2)/2. Percent tumor growth inhibition (TGI) values were calculated on the final day of the study for CX-4945–treated compared to vehicle-treated mice and were calculated as 100 × {1 ? [(TreatedFinal day ? TreatedDay 1)/(ControlFinal day ? ControlDay 1)]}. The significance of the differences between the treated versus vehicle groups were determined using 1-way ANOVA [1]. |
| In vitro | The antiproliferative activity of Silmitasertib (CX-4945) against cancer cells correlated with expression levels of the CK2α catalytic subunit. CX-4945 caused cell-cycle arrest and selectively induced apoptosis in cancer cells relative to normal cells. In models of angiogenesis, CX-4945 inhibited human umbilical vein endothelial cell migration, tube formation, and blocked CK2-dependent HIF-1α transcription in cancer cells [1]. Adding CX-4945 after bortezomib treatment, prevented leukemic cells from engaging a functional UPR in order to buffer the bortezomib-mediated proteotoxic stress in ER lumen. Bortezomib/CX-4945 treatment inhibited NF-κB signaling in T-ALL cell lines and primary cells from T-ALL patients, but, in B-ALL cells the drug combination activated NF-κB p65 pro-apoptotic functions [2]. CX-4945 was found to potently inhibit endogenous intracellular CK2 activity with an IC50 of 0.1 μM in Jurkat cells. CX-4945 induced dephosphorylation of Akt(S129) and a rapid dephosphorylation of the Akt substrate p21(T145). CX-4945 induced apoptosis in multiple cancer cell lines [3]. |
| In vivo | When administered orally in murine xenograft models, CX-4945 was well tolerated and demonstrated robust antitumor activity with concomitant reductions of the mechanism-based biomarker phospho-p21 (T145) [1]. Mice bearing subcutaneous PC3 tumors were treated with CX-4945 (25 mg/kg, 50 mg/kg, and 75 mg/kg, po, bid). Tumor growth inhibition compared to vehicle-treated control, and a dose-responsive efficacy was observed. Last, CX-4945 was well tolerated in mice as assessed by minimal changes in body weight during the course of treatment compared to vehicle control [3]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | H2O : < 1 mg/mL (insoluble or slightly soluble)
Ethanol : < 1 mg/mL (insoluble or slightly soluble)
DMSO : 50 mg/mL (142.95 mM)
|
| Keywords | Inhibitor | inhibit | Casein Kinase | CX4945 | Silmitasertib | Autophagy | CX 4945 |
| Inhibitors Related | Stavudine | Xylitol | Myricetin | Sodium 4-phenylbutyrate | Hydroxychloroquine | Guanidine hydrochloride | Taurine | Curcumin | Oxyresveratrol | Paeonol | Naringin | Gefitinib |
| Related Compound Libraries | Bioactive Compound Library | Kinase Inhibitor Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Inhibitor Library | Anti-Aging Compound Library | Bioactive Compounds Library Max | Wnt/Hedgehog/Notch Compound Library | Anti-Cancer Active Compound Library | Anti-Cancer Drug Library |

 United States
United States