Product Number: R010004
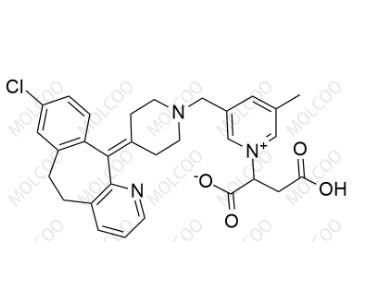
English Name: Rupatadine EP Impurity A
English Alias: 3-carboxy-2-(3-((4-(8-chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)methyl)-5-methylpyridin-1-ium-1-yl)propanoate
CAS Number: 1354055-68-7
Molecular Formula: C30H30ClN3O4
Molecular Weight: 532.03
Advantages: Rupatadine EP Impurity A is synthesized via advanced processes and multi-stage purification, featuring high purity and strong stability. Structural confirmation and content determination are conducted by precise analytical methods like NMR and HRMS, ensuring accurate chemical structure and uniform composition. It provides a reliable reference standard for drug R&D and quality control, effectively guaranteeing the accuracy and reproducibility of experimental data.
Applications: Mainly used for impurity analysis, quality control, and EP compliance verification of Rupatadine. In R&D, it determines impurity content and evaluates impacts on efficacy and safety; in production, as an EP reference standard, it detects impurity levels to ensure compliance with EP and other international regulations. It also applies to drug stability studies and degradation pathway analysis.
Background Description: As a highly effective anti-allergic drug, impurity control for Rupatadine is central to its quality system. The European Pharmacopoeia (EP) imposes strict limits on drug impurities. Rupatadine EP Impurity A, as a key impurity, may affect drug purity and efficacy, making its precise control an important step to ensure drug safety and a necessary condition for international drug registration.
Research Status: Current research focuses on developing highly sensitive detection methods and exploring generation mechanisms. Researchers use UPLC-MS/MS for trace analysis and optimize synthesis routes to reduce its formation during production. Meanwhile, studies on its effects on drug stability and biological activity are progressing, providing a theoretical basis for formulating reasonable impurity control strategies.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China