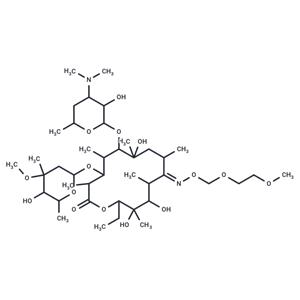
| Name | Roxithromycin |
| Description | Roxithromycin (RU-28965) is a semi-synthetic macrolide antibiotic derived from erythromycin, exhibiting both antibacterial and anti-malarial properties. |
| Kinase Assay | The half-maximal inhibitory concentrations (IC50s) of Fenofibrate, statins (atorvastatin, lovastatin, pravastatin, simvastatin and simvastatin acid, the active form of simvastatin) and glipizide for recombinant human CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 are determined using fluorometric CYP450 inhibition assays. Briefly, the drugs are dissolved in methanol or acetonitrile. In 96 well assay plates, the drugs are diluted to a series of concentrations in a solution containing cofactors including NADP+ (final concentration 1.3 mM), MgCl2 (final concentration 3.3 m M), glucose-6-phosphate (G6P, final concentration 3.3 mM) and glucose 6-phosphate dehydrogenase (final concentration 0.4 U/mL). The mixture is pre-incubated at 37°C for 10 min. The enzymes and fluorogenic substrates are diluted to desired concentrations in sodium phosphate reaction buffer (pH 7.4, final concentration 200 mM) and mixed. Reactions are initiated with addition of the enzyme and substrate mixture to the cofactor and drug mixture. The final reaction volume of all assays is 200 μL. After incubating at 37°C for a pre-specified period of time (15 to 45 min), the reactions are stopped with addition of 75 μL quenching solution (0.5 M Tris base or 2N NaOH). Fluorescence is determined using a BioTek Synergy 2 fluorescence reader. Each of the drugs is tested at eight concentrations in duplicate. To estimate IC50s, percent of inhibition is calculated using net fluorescence that is corrected for the background. The values of percent of inhibition are then fitted to a three or four parameter log-logistic model[1]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | 10% DMSO+40% PEG300+5% Tween 80+45% Saline : 2 mg/mL (2.39 mM), Sonication is recommended.
Ethanol : 154 mg/mL (183.98 mM), Sonication is recommended.
H2O : < 1 mg/mL (insoluble or slightly soluble)
DMSO : 252.5 mg/mL (301.65 mM), Sonication is recommended.
|
| Keywords | RU28965 | RU 28965 | Roxithromycin | Inhibitor | inhibit | Bacterial | Antibiotic | 50S ribosome |
| Inhibitors Related | Neomycin sulfate | Aceglutamide | Tamoxifen | Adipic dihydrazide | Levulinic acid | D(+)-Raffinose pentahydrate | Sulfamethoxazole sodium | Terbinafine hydrochloride | Ethyl linoleate | Dimethyl sulfoxide | Sodium diacetate | BES |
| Related Compound Libraries | FDA-Approved & Pharmacopeia Drug Library | Anti-Parasitic Compound Library | Bioactive Compound Library | Drug Repurposing Compound Library | Microbial Natural Product Library | Anti-Cancer Approved Drug Library | Immunology/Inflammation Compound Library | Bioactive Compounds Library Max | Covalent Inhibitor Library | Anti-Infection Compound Library | Human Metabolite Library | Anti-Cancer Drug Library |

 United States
United States