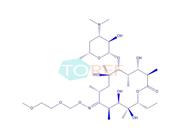
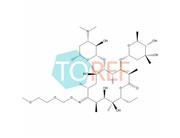
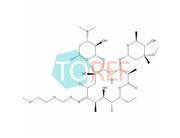
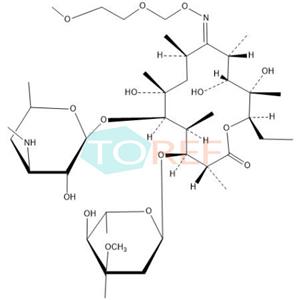
Product Name: Roxithromycin EP Impurity F
CAS No. 118267-18-8
Product Code: REF-R05007
MF: C40H74N2O15
MW: 823.03
Purity: 95%+
Chemical name:(3R,4S,5S,6R,7R,9R,11S,12R,13S,14R,E)-14-ethyl-7,12,13-trihydroxy-4-(((2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-6-(((2S,3R,4S,6R)-3-hydroxy-6-methyl-4-(methylamino)tetrahydro-2H-pyran-2-yl)oxy)-10-(((2-methoxyethoxy)methoxy)imino)-3,5,7,9,11,13-hexamethyloxacyclotetradecan-2-one
Appearance: dark
Storage conditions: 2-8 ℃
Synthesis of Roxithromycin EP Impurity F
Roxithromycin EP Impurity F Reference Standards,
Complex Impurity Of Roxithromycin EP Impurity F,
Chemical Standards of Roxithromycin EP Impurity F,
Characterization Of Unknown Impurities of Roxithromycin EP Impurity F,
Structure Profiling of Roxithromycin EP Impurity F
Identification of Roxithromycin EP Impurity F
Isolation & Purification of Impurity of Roxithromycin EP Impurity F
Difficult of Roxithromycin EP Impurity F
Instability of Roxithromycin EP Impurity F
Low-assay of Roxithromycin EP Impurity F
Complex Impurity Of Chemical Standards of Roxithromycin EP Impurity F
TOSUN PHARM was established in 1999. In China, it is a collection of drugs, APIs, reference listed drugs, impurities, excipients, intermediates import and export, import registration services, generic drug research and development services, innovative drugs and high-end FDF technology transfer, A group company integrating marketing, academic promotion and cooperative production. With a global procurement, R&D and marketing network, it can quickly meet the needs of product R&D, production and market sales of Chinese and global pharmaceutical companies.
Hot Tags: Roxithromycin EP Impurity F, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock




 China
China