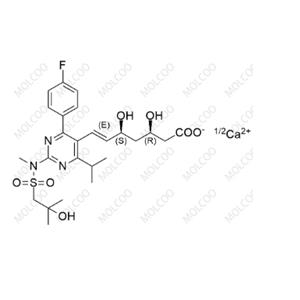
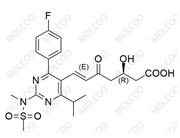
Rosuvastatin Impurity NEW
$0.00 10mg
$0.00 30mg
$0.00 100mg
- Min. Order10mg
- Purity98%+
- Cas No1714147-47-3
- Supply Ability20g
- Update time2025-04-09

Moxin Chemicals
1YR
 China
China
Chemical Properties
| Product Name | Rosuvastatin Impurity |
| CAS No | 1714147-47-3 |
| EC-No | |
| Min. Order | 10mg |
| Purity | 98%+ |
| Supply Ability | 20g |
| Release date | 2025/04/09 |