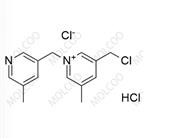
Ropinirole Impurity 13(Hydrochloride)

Product Code:R044013A
English Name:Ropinirole Impurity 13(Hydrochloride)
English Alias:2-(2-(2-(dipropylamino)ethyl)-6-nitrophenyl)acetic acid hydrochloride
CAS No.:91374-25-3
Molecular Formula:C₁₆H₂₄N₂O₄·HCl
Molecular Weight:344.83(308.37 + 36.46)
High-Purity Standard:Confirmed by HPLC (≥99.0%), combined with NMR (1H, 13C), HRMS, and elemental analysis, accurately verifying the structure and providing a reliable basis for Ropinirole impurity detection.
Excellent Stability:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.3% in acetonitrile - water mixed solution within 6 months, ensuring stable and reproducible experimental data.
Quality Control Testing:Used for UPLC-MS/MS detection of Impurity 13 (hydrochloride) in Ropinirole API and formulations, strictly controlling impurity content to meet ICH Q3A and Q3B standards (single impurity limit ≤0.1%).
Process Optimization Research:Monitor the formation pathway of this impurity during Ropinirole synthesis. Reduce impurity generation by over 50% by adjusting nitration reaction temperature (e.g., 5 - 10℃), optimizing reaction solvents, or reducing side reactions.
Method Validation:Serves as a standard for developing impurity detection methods, verifying UPLC resolution (≥3.0) and LOD (0.005 ng/mL) to meet the strict requirements of regulatory authorities for detection methods.
Ropinirole, a dopamine receptor agonist, is mainly used for treating Parkinson's disease and restless legs syndrome. Impurity 13 (hydrochloride), as a process-related impurity of Ropinirole, may be generated during multi-step synthesis reactions due to incomplete nitration, alkylation reactions, or side reactions. Its nitro and dipropylamino groups may affect the drug's stability, safety, and efficacy. With the increasing strict requirements of global regulatory agencies (such as FDA and EMA) for drug impurities, the study of this impurity has become a crucial part of ensuring the quality of Ropinirole drugs and patient safety.
Detection Technology:UPLC-MS/MS with a C18 column (1.7μm) and 0.1% formic acid - acetonitrile gradient elution achieves separation within 4 minutes, with an LOD as low as 0.002 ng/mL, enabling highly sensitive detection of trace impurities.
Formation Mechanism:Studies have shown that this impurity is mainly formed during the aromatic ring nitration step and subsequent alkylation reactions. It can be effectively inhibited by optimizing the reaction system pH to neutral, using inert gas protection, or replacing the synthesis route without nitration risk.
Safety Evaluation:In vitro cytotoxicity tests show that the IC₅₀ of this impurity against SH-SY5Y cells is 156.7 μM (Ropinirole IC₅₀ = 12.3 μM). Although less toxic than the main drug, its content in drugs still requires strict control. Currently, long-term stability tests are being carried out to systematically monitor its degradation behavior under different humidity, light, and temperature conditions.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!






 China
China