Rizatriptan EP Impurity A(Dihydrochloride)
Product Code:R047002A
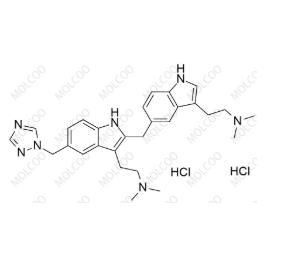
English Name:Rizatriptan EP Impurity A(Dihydrochloride)
English Alias:2-(5-((1H-1,2,4-triazol-1-yl)methyl)-2-((3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)methyl)-1H-indol-3-yl)-N,N-dimethylethanamine dihydrochloride
CAS No.:[Not Available]
Molecular Formula:C₂₈H₃₅N₇·2HCl
Molecular Weight:542.54(469.62 + 2×36.46)
Ultra-High Purity:Confirmed by HPLC (≥99.5%), combined with NMR (1H, 13C), HRMS, and elemental analysis, ensuring accuracy and reliability in impurity analysis.
Excellent Stability:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.1% in acetonitrile-water (1:1) solution within 6 months, ensuring high reproducibility of experimental data.
Quality Control Testing:Used for UPLC-MS/MS detection of EP Impurity A (dihydrochloride) in Rizatriptan API and formulations, controlling impurity content to meet EP standards (single impurity limit ≤0.1%).
Process Optimization Research:Monitors the formation pathway of this impurity during Rizatriptan synthesis, reducing generation by over 50% by adjusting condensation reaction temperature (e.g., 80-90℃) and reaction time.
Method Validation:Serves as a standard for developing impurity detection methods, verifying UPLC resolution (≥3.0) and LOD (0.005 ng/mL).
Rizatriptan, a 5-hydroxytryptamine receptor agonist, is used for treating migraines. Impurity A (dihydrochloride), as an EP-specified impurity of Rizatriptan, may be generated during multi-step synthesis due to incomplete alkylation of the indole ring or connection of the triazole ring. Its complex bis-indole structure and dimethylamino group may affect drug stability and safety. With the stricter control requirements of EMA for European Pharmacopoeia impurities, the study of this impurity has become a key link in drug registration and production.
Detection Technology:UPLC-MS/MS with a C18 column (1.7μm) and 0.1% formic acid-acetonitrile gradient elution achieves separation within 6 minutes, with an LOD of 0.002 ng/mL, meeting the needs of trace analysis.
Formation Mechanism:Studies have shown that this impurity is formed by the reaction of 3-(2-dimethylaminoethyl)indole with 1,2,4-triazole methylation reagents under alkaline conditions (e.g., potassium carbonate catalysis). Optimizing the solvent system (such as using DMF instead of ethanol) can inhibit side reactions.
Safety Evaluation:In vitro cytotoxicity experiments show that the IC₅₀ of this impurity against SH-SY5Y cells is 189.3 μM (Rizatriptan IC₅₀ = 12.6 μM). Although less toxic than the main drug, its content in drugs still needs to be strictly controlled. Currently, long-term stability tests are being conducted to monitor its degradation behavior.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!