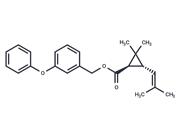
| Name | Rimonabant |
| Description | Rimonabant (SR141716) is an inverse agonist for the cannabinoid receptor CB1. It is an anorectic anti-obesity drug produced and marketed by Sanofi-Aventis. Its main avenue of effect is a reduction in appetite. Rimonabant is the first selective CB1 receptor blocker to be approved for use anywhere in the world. Rimonabant is approved in 38 countries including the E.U., Mexico, and Brazil. It was rejected for approval for use in the United States. |
| Cell Research | Raw 264.7 cells (2 × 106 /well) in 12-well plates are rinsed with PBS and refed culture media supplemented with varying amounts of Rimonabant 1 hour prior to supplementation with 7-ketocholesterol (7KC). All wells are adjusted to receive equal amounts of vehicle. Following 16-hour incubation, caspase-3 and caspase 3-like activity are determined using a fluorogenic substrate (Ac-DEVD-AFC) and a spectrofluorometer equipped with a microplate reader. (Only for Reference) |
| Kinase Assay | Radioligand Binding Assay: Human CB1 and CB2 stably transfect HEK 293 cells and cell membrane is purified. 0.2-8 μg of the purified membrane is incubated with 0.75 nM [3H] CP55,940 and Rimonabant in the incubation buffer (50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, 0.3%BSA, pH 7.4). The non-specific binding is defined in the presence of 1 μM of CP55,940. The reactions are incubated for one and a half hours at 30 °C in Multiscreen. The reactions are terminated by manifold filtration and washed four times with ice-cold wash buffer (50 mM Tris, pH 7.4, 0.25% BSA).The radioactivity bound to the filters is measured by Topcount. The IC50 is determined as the concentration of Rimonabant required to inhibit 50% of the binding of [3H] CP55,940 and calculated by non-linear regression. |
| In vitro | Rimonabant dose-dependently reduces ACAT activity in Raw264.7macrophages with IC50 of 2.9 μM and isolated peritoneal macrophages. Rimonabant inhibits ACATactivity in intact CHO-ACAT1 and CHO-ACAT2 cells and in cell-free assays with approximately equal efficiency with IC50 of 1.5 μM and 2.2 μM for CHO-ACAT1 and CHO-ACAT2, respectively. Consistent with ACAT inhibition, Rimonabant treatment blocks ACAT dependent processes in macrophages, oxysterol-induced apoptosis and acetylated-LDL induced foam cell formation. [2] Rimonabant antagonizes the inhibitory effects of cannabinoid receptor agonists on both mouse vas deferens contractions and adenylyl cyclase activity in rat brain membranes in a concentration-dependent manner. [3] Rimonabant significantly reduces cell growth and induces cell death of human colorectal cancer cells (DLD-1, CaCo-2 and SW620). Rimonabant is able to alter cell cycle distribution in all the cell lines tested. Particularly, Rimonabant produces a G2/M cell cycle arrest in DLD-1 cells without inducing apoptosis or necrosis. [4] |
| In vivo | Rimonabant is administered intraperitoneally or orally potently and dose-dependently antagonize classical pharmacological and behavioural effectos of cannabinoid receptor agonists. [3] In the mouse model of azoxymethane-induced colon carcinogenesis, Rimonabant significantly decreased aberrant crypt foci (ACF) formation, which precedes colorectal cancer. [4] Rimonabant (10 mg/kg by gavage) is fed for 2 weeks to 3-month-old male obese Zucker rats as an impaired glucose tolerance model and for 10 weeks to 6-month-old male obese Zucker rats as a model of the metabolic syndrome. RANTES (Regulated upon Activation, Normal T cell Expressed, and Secreted) and MCP-1 (monocyte chemotactic protein-1) serum levels are increased in obese vs lean Zucker rats and significantly reduced by long-term treatment with Rimonabant, which slowes weight gain in rats with the metabolic syndrome. Neutrophils and monocytes are significantly increased in young and old obese vs lean Zucker rats and lowered by Rimonabant. Platelet-bound fibrinogen is significantly enhanced in obese vs lean Zucker rats of both age, and is reduced by Rimonabant. Platelets from obese rats are more sensitive to thrombin-induced aggregation and adhesion to fibrinogen, which are both attenuated by Rimonabant therapy. [5] |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | H2O : <1 mg/mL
Ethanol : 2 mg/mL (4.31 mM)
DMSO : 24 mg/mL (51.7 mM)
|
| Keywords | SR-141716 | SR 141716 | Rimonabant | Inhibitor | inhibit | Cannabinoid Receptor | Bacterial |
| Inhibitors Related | Neomycin sulfate | Dehydroacetic acid sodium | Ampicillin sodium | Methyl anthranilate | Kanamycin sulfate | G-418 disulfate | Sulfamethoxazole sodium | Metronidazole | Doxycycline | Dimethyl sulfoxide | 2,3-Butanediol | Crystal Violet |
| Related Compound Libraries | Target-Focused Phenotypic Screening Library | Anti-Neurodegenerative Disease Compound Library | Bioactive Compound Library | Anti-Alzheimer's Disease Compound Library | Drug Repurposing Compound Library | Inhibitor Library | Metabolism Compound Library | Orally Active Compound Library | Bioactive Compounds Library Max | GPCR Compound Library | Anti-Cancer Compound Library | Anti-Infection Compound Library |

 United States
United States