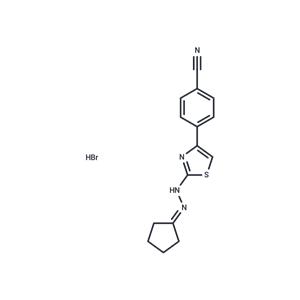
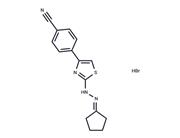
| Name | Remodelin |
| Description | Remodelin is an effective and specific inhibitor of the acetyl-transferase protein NAT10. |
| Kinase Assay | GRK5 and urea-washed bovine rod outer segments (ROS) are mixed in the dark in buffer containing 20 mM HEPES, pH 7.5, 4 mM MgCl2, and 2 mM EDTA and incubated for 35 min at room temperature. The reaction mixtures are exposed to ambient fluorescent light for 1 min prior to initiation of the reaction by addition of ATP (with [γ-32P]ATP) to a final concentration of 1 mM. Final concentration of GRK5 is 100 nM and ROS is between 0.75 and 24 μM. Reactions are initiated at room temperature, and samples are taken at 2-5 min and then quenched with SDS-PAGE loading dye. Proteins are separated using SDS-PAGE, gel is dried, and the incorporation of γ-32P is detected using a phosphor storage screen. Rates at 0 min are plotted against the ROS concentration, and Vmax and Kmvalues are determined using the Michaelis-Menten equation. Vmax of each curve is normalized to the Vmax of GRK5561 run in parallel. Melting point determinations in response to 200 μM CCG215022 are performed in 20 mM HEPES, pH 7.0, 5 mM MgCl2, 2 mM DTT, 1 mM CHAPS at a final GRK5 concentration of 0.2 mg/mL and 100 μM anilinonaphthalene-8-sulfonic acid using a ThermoFluor plate reader. Melting points of GRK5 variants are assayed in a buffer containing 20 mM HEPES, pH 8.0, 200 mM NaCl, 2 mM DTT, 2.5 mM MgCl2, and 0.1 mM anilinonaphthalene-8-sulfonic acid with or without 5 mM ATP. Final GRK5 concentration for these assays is 0.1 mg/mL[1]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 25 mg/mL (88.54 mM)
|
| Keywords | HAT | inhibit | Inhibitor | HATs | Remodelin | Histone Acetyltransferase |
| Inhibitors Related | Anacardic Acid | Remodelin hydrobromide | Acetaminophen | MG 149 | C646 | Curcumin | CTX-0124143 | CPI-637 | WM-1119 | Naphthol AS-E | PU139 | NEO2734 |
| Related Compound Libraries | Histone Modification Compound Library | Bioactive Compound Library | Epigenetics Compound Library | Chromatin Modification Compound Library | Inhibitor Library | Stem Cell Differentiation Compound Library | NO PAINS Compound Library | Bioactive Compounds Library Max | Anti-Hypertension Compound Library |

 United States
United States