Product Number: E038010A
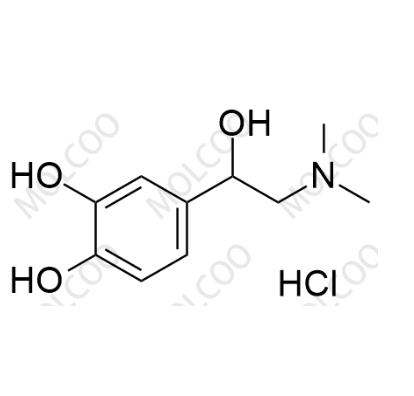
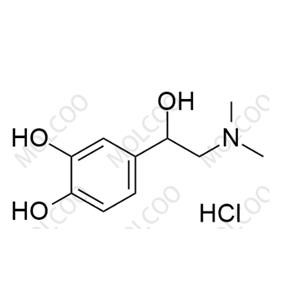
English Name: rac-N-Methyl Epinephrine (Hydrochloride)
English Alias: 4-(2-(dimethylamino)-1-hydroxyethyl)benzene-1,2-diol hydrochloride
CAS Number: 62-22-6
Molecular Formula: C₁₀H₁₅NO₃.HCl
Molecular Weight: 197.23 (free base); 36.46 (hydrochloride moiety)
As a racemic form of N-methyl epinephrine hydrochloride, this compound has the following advantages:
Well-defined with distinct functional groups: Contains a catechol structure (o-dihydroxybenzene), 2-(dimethylamino)-1-hydroxyethyl side chain, and hydrochloride group. Unlike epinephrine (with monomethylamino), its dimethylamino weak basicity and catechol polarity create unique physicochemical properties, enabling precise separation via HPLC/ion-exchange chromatography as a specific marker;
High stability and water solubility: The hydrochloride form significantly enhances water solubility (compared to free base), and the catechol structure remains stable under dark, acidic conditions, suitable for long-term storage as a solution standard;
High detection sensitivity: Catechol groups show strong UV absorption (around 280nm), combined with m/z 198 [M+H]⁺ (free base) enabling ppb-level analysis via LC-MS, compatible with catecholamine detection systems.
Pharmaceutical quality control: Used as an impurity reference standard to quantify N-methyl analogs in epinephrine-related drugs, ensuring purity by evaluating methylation byproducts;
Pharmacological research tool: Investigating structure-activity relationships of catecholamines, comparing receptor binding effects of N-methyl substitution to guide drug design;
Method validation: Validating accuracy of catecholamine assays, particularly for quantifying metabolites in biological samples (plasma, urine).
N-Methyl epinephrine is a methylation metabolite of epinephrine, catalyzed by catechol-O-methyltransferase (COMT) in vivo. Its racemic hydrochloride form (rac-N-Methyl Epinephrine (Hydrochloride)) can be chemically synthesized. Due to structural similarity to epinephrine, it may exist as a synthetic byproduct or in vivo metabolite, making its detection critical for understanding drug efficacy and metabolism.
Current research focuses on:
Analytical method validation: Developing UPLC assays with C18 columns for separation, achieving 0.05 ppb detection limits from related catecholamines;
Metabolic mechanisms: Studying COMT-catalyzed N-methylation efficiency via in vitro assays to link metabolite levels with physiological states (stress, disease);
Pharmacological evaluation: Comparing α/β receptor agonism with epinephrine to clarify reduced activity from N-methyl substitution;
Synthesis optimization: Improving methylation steps to enhance purity, supporting large-scale production as a reference standard.





 China
China