- Product Information
Product Number: D028005
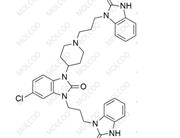
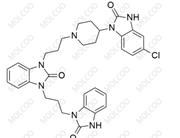
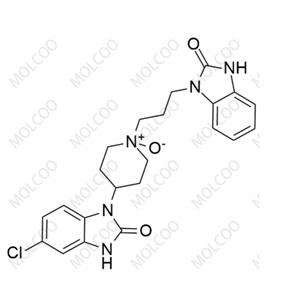
English Name: rac-Domperidone EP Impurity C
English Alias: Domperidone N-Oxide; 4-(5-chloro-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)-1-(3-(2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)propyl)piperidine 1-oxide
CAS Number: 118435-03-3
Molecular Formula: C₂₂H₂₄ClN₅O₃
Molecular Weight: 441.91
As a European Pharmacopoeia (EP) standard impurity of domperidone, this compound has the following advantages:
Well-defined structure compliant with EP standards, directly applicable to quality testing of domperidone APIs and formulations to ensure consistency with international pharmacopoeia requirements;
As an N-oxide impurity, it can be used to analyze the by-product formation mechanism of oxidation reactions during domperidone synthesis or storage, optimizing processes to control N-oxide impurity generation;
Helps study the impact of N-oxide structures on drug stability and safety (N-oxides may have different pharmacological activities or toxicities) to provide a scientific basis for impurity control strategies.
Quality Control: Used as an EP standard impurity reference for system suitability tests in HPLC and other detection methods to verify whether the content of this impurity in domperidone meets EP limits;
Drug Development: In generic drug research, used to compare the impurity profile of the original drug to ensure quality consistency between the generic and original drug;
Stability Studies: Investigating the formation rate of this impurity under light and high-temperature conditions to evaluate its impact on domperidone formulation stability and assist in establishing storage conditions.
Domperidone is a dopamine receptor antagonist clinically used for treating dyspepsia, nausea, and vomiting. The piperidine nitrogen atom in its molecular structure is prone to oxidation reactions in the presence of oxidants or during storage, generating N-oxide impurities (such as Impurity C). The European Pharmacopoeia (EP) has clear limits for this impurity, making research on it a necessary part of international quality control for domperidone.
Current research focuses on:
Detection Method Optimization: Using EP-recommended HPLC-UV or LC-MS combined techniques to improve the detection sensitivity and specificity of this impurity;
Synthesis Process Improvement: Reducing N-oxide by-product generation by adjusting antioxidant conditions in the reaction system (such as inert gas protection, antioxidant addition);
Toxicological Evaluation: Studying the potential toxicity of N-oxide structures through in vitro cell experiments to provide data support for establishing safe limits;
Stability Prediction: Establishing a formation kinetics model for this impurity based on accelerated test data to predict the shelf life of domperidone formulations.





 China
China