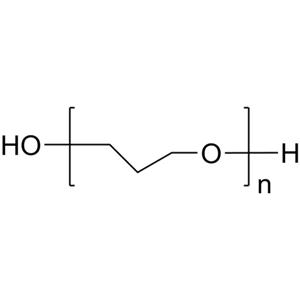
Polyethylene Glycol 400 (PEG400) is commonly used as a solvent and is a colorless, viscous liquid at room temperature. It can fully dissolve substances such as vitamin C, vitamin E, and hydroquinone, and can completely dissolve titanium dioxide at high temperatures of 100°C. PEG400 is a non-ionic, inert solvent that fully dissolves undecylenic acid and its zinc salt at 100°C, remaining stable at room temperature without reacting with active substances like transdermal agents and thus not affecting their efficacy. Polyethylene Glycol, commonly referred to as "PEG," is a polymer mixture formed through the intermolecular dehydration condensation of ethylene glycol. Its chemical formula is HOCH2(CH2OCH2)nCH2OH, where n is greater than 4. The average molecular weight ranges from 200 to 7000. The number following the product name Polyethylene Glycol indicates the average molecular weight. For example, Polyethylene Glycol-400 has an average molecular weight of around 400. It is a colorless viscous liquid or a white solid, low in toxicity, soluble in water and many organic solvents, readily soluble in aromatic hydrocarbons, slightly soluble in aliphatic hydrocarbons. It does not hydrolyze over time, is heat-stable, and unreactive with many chemicals. It can serve as a plasticizer, softener, humectant, lubricant, solvent, adhesive, and is also used for fragrance mixing and pharmaceutical formulations. Polyethylene Glycol-400: n is about 8.2–9.1, with a molecular weight of 380–420, a colorless, slightly hygroscopic viscous liquid with a distinctive odor. Its specific gravity is 1.110–1.140 (25/25℃), with a freezing point of 4–8℃. The viscosity is (6.8–8.0)×10^-2 Pa·s (at 116.6K).

 China
China