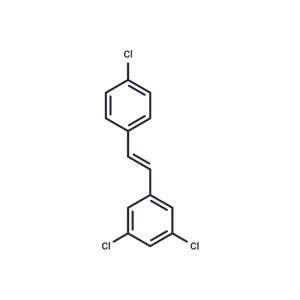
| Name | PDM2 |
| Description | PDM 2 is a potent and selective aryl hydrocarbon receptor (AhR) antagonist. |
| In vitro | In a previous screening study, it was found that the replacement of resveratrol hydroxyls by the same substituent produced compounds with the following order of affinity: OH (resveratrol) , OMe < F < CF3 < Cl (PDM2). PDM2 exhibited a Ki of 1.25 for AhR and no affinity for ER, indicating that replacement of hydroxyl with chloride could abolish binding on ER and dramatically increase the affinity for AhR. Moreover, among its analogs PDM2 was the most potent AhR antagonists in this series, being 10-fold more efficient than resveratrol. PDM2, devoid of measurable affinity for ER, did not display any effect on ER-driven transcription. Therefore, PDM2 was considered as a selective AhR modulator with regard to ER. In addition, its trimethoxylated derivatives and 3,5-methoxy derivatives were able to induce cytotoxicity at doses lower than 100 nM, which was consistent with previous data. 3,5-Methoxy derivatives, however, only showed cytotoxicity at concentrations higher than 10 μM [1]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 50 mg/mL (176.32 mM), Sonication is recommended.
|
| Keywords | PDM-2 | PDM2 | PDM 2 | Inhibitor | inhibit | Aryl Hydrocarbon Receptor | AhR |
| Inhibitors Related | Nimodipine | Carbidopa monohydrate | Diosmin | Prochloraz | (-)-Perillaldehyde | Benzyl butyl phthalate | L-Kynurenine | Indole-3-carbinol | MeBIO | Leflunomide | Mexiletine hydrochloride | PD98059 |
| Related Compound Libraries | Nonsteroidal Anti-Inflammatory Compound Library | Nuclear Receptor Compound Library | Bioactive Compound Library | Inhibitor Library | NO PAINS Compound Library | Immunology/Inflammation Compound Library | Bioactive Compounds Library Max | Anti-Metabolism Disease Compound Library | Transcription Factor-Targeted Compound Library |

 United States
United States