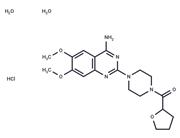
| Name | Ondansetron hydrochloride dihydrate |
| Description | Ondansetron hydrochloride dihydrate (GR 38032) is a competitive serotonin type 3 receptor antagonist. It is effective in the treatment of nausea and vomiting caused by cytotoxic chemotherapy drugs, including cisplatin, and has reported anxiolytic and neuroleptic properties. |
| Cell Research | Cells are plated in 60-mm Petri dishes (2×105 per dish) and cultured in serumcontained medium for 24 h. The next day the medium is removed and serum-contained medium with or without Dox (0.1 or 0.5 μg/ml) and Ond (10 or 30 μg/ml) in combination or respectively, is added. After 3 days, the cells are washed twice with PBS, trypsinized, and counted by using the trypan blue exclusion method.(Only for Reference) |
| In vitro | Ondansetron is a potent, highly selective, competitive antagonist at 5-HT3 receptors. It demonstrates some affinity to other receptor subtypes, including 5-HT1B, 5-HT1C, 5-HT4, opioid, and 1-adrenergic receptors, and to the μ-opioid receptor. However, ondansetron has 1000:1 selectivity toward 5-HT3 receptors[1]. Ondansetron is found to be the most potent HERG-channel blocker among several 5-HT3 antagonists, with an IC50 of 810 nM and has been reported to block Na+ channels[2]. |
| In vivo | Ondansetron(Ond) is well tolerated and its side effects are mild. Ond acts on the CNS as well as on the peripheral nervous system (PNS). Ondansetron is available both for oral and intravenous administration. The bioavailability of orally administered ondansetron is only 60%. The low bioavailability is due to a significant first-pass metabolism. The peak plasma concentration of ondansetron is usually reached at approximately 1.5 h after oral administration. A major portion of this drug, about 75%, is bound to plasma proteins. Ondansetron is currently used to prevent and treat nausea and vomiting associated with chemotherapy, radiation treatment and general anesthesia. It has been shown to inhibit GABA and glycine receptor activity in animal models[1]. Therapeutic dose of Ond allows delivery of significantly higher amounts of Dox to the brain tissue in vivo, which is otherwise disallowed by the BBB. The rate of penetration of the blood-brain barrier by Ond is very low[3]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | Ethanol : 38 mg/mL (103.86 mM), Sonication is recommended.
10% DMSO+40% PEG300+5% Tween 80+45% Saline : 1 mg/mL (2.73 mM), Sonication is recommended.
H2O : 67 mg/mL (183.13 mM), Sonication is recommended.
DMSO : 10 mg/mL (27.33 mM), Sonication is recommended.
|
| Keywords | vomiting | SN-307 | SN307 | SN 307 hydrochloride dihydrate | Serotonin Receptor | radiation | Ondansetron hydrochloride dihydrate | Ondansetron hydrochloride Dihydrate | Ondansetron Hydrochloride | NSC-665799 | NSC665799 | nausea | Inhibitor | inhibit | GR-38032 | GR38032 | GR 38032 hydrochloride dihydrate | cancer surgery | cancer chemotherapy | 5-hydroxytryptamine Receptor | 5HTReceptor | 5-HT3 | 5-HT Receptor | 5HT Receptor |
| Inhibitors Related | Alverine citrate | Olanzapine | Dapoxetine hydrochloride | Clozapine N-Oxide | Mirtazapine | D-Menthol | Amitriptyline hydrochloride | Cloperastine hydrochloride | Trazodone hydrochloride | Mianserin hydrochloride | Fluoxetine hydrochloride | Cinchonidine |

 United States
United States