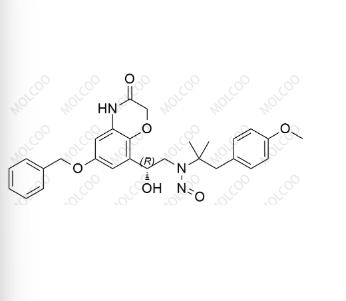
Olodaterol Nitroso Impurity;C28H31N3O6
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Code:O027049
English Name:Olodaterol Nitroso Impurity 49
English Alias:(R)-N-(2-(6-(benzyloxy)-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-8-yl)-2-hydroxyethyl)-N-(1-(4-methoxyphenyl)-2-methylpropan-2-yl)nitrous amide
CAS No.:Not provided (to be supplemented)
Molecular Formula:C₂₈H₃₁N₃O₆
Molecular Weight:505.56
High-Purity Reference Standard:Confirmed by HPLC (≥99.0%), NMR (1H, 13C), HRMS, and elemental analysis, suitable for Olodaterol impurity analysis.
Stability Assurance:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.3% in acetonitrile-methanol within 6 months.
Quality Control Testing:Used for UPLC-MS/MS detection of Impurity 49 in Olodaterol API and formulations, meeting ICH Q3A standards (single impurity limit ≤0.1%).
Process Optimization:Monitors impurity formation during synthesis, reducing generation by >40% by adjusting nitrosation temperature (e.g., 0-5℃) and reaction time.
Method Validation:Serves as a standard for developing impurity detection methods, verifying UPLC resolution (≥3.0) and LOD (0.01 ng/mL).
Olodaterol, a long-acting β₂-adrenergic receptor agonist, is used for treating COPD. Impurity 49, a nitroso-related impurity, may originate from nitrosation side reactions of amino groups during synthesis. Its nitroso, hydroxyl, and benzoxazine groups may affect drug stability and receptor binding. With stricter requirements for respiratory drug impurities, studying this impurity is crucial for ensuring drug quality.
Detection Technology:UPLC-MS/MS with C18 column (1.7μm) and 0.1% formic acid-acetonitrile gradient elution separates impurities within 10 min, with LOD of 0.005 ng/mL.
Formation Mechanism:Formed by nitrosation of amino-containing intermediates with sodium nitrite under acidic conditions (e.g., acetic acid system); optimizing acid concentration and reaction time inhibits side reactions.
Safety Evaluation:In vitro cytotoxicity shows IC₅₀ of 185.7 μM against BEAS-2B cells (Olodaterol IC₅₀=9.2 μM). Although less toxic than the main drug, content control is required, and long-term stability testing is in progress.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!