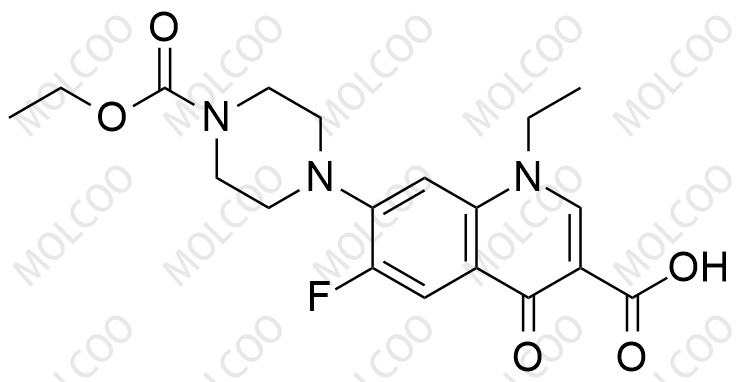
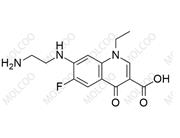
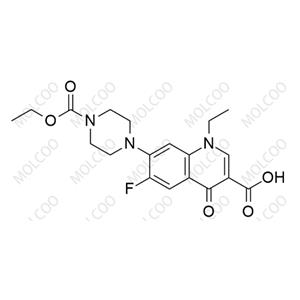
Norfloxacin EP Impurity H
Norfloxacin Impurity Reference Standards
Norfloxacin impurity reference standards are crucial tools in the fields of drug research and development, quality control, and pharmaceutical testing. As a widely used antimicrobial agent, the purity and safety of Norfloxacin are of utmost importance. To ensure the quality of Norfloxacin products, accurate identification and quantitative analysis of Norfloxacin and its impurities are essential steps.
Our Norfloxacin impurity reference standards cover a variety of key impurities, including Norfloxacin impurity A, among others. These impurity reference standards have been meticulously prepared and undergone rigorous quality control to ensure their purity and chemical characteristics meet international and industry standards.
With Norfloxacin impurity reference standards, you can:
Accurately Identify Impurities: By comparing with impurity reference standards, you can quickly and accurately identify impurity components in pharmaceutical products.
Quantitatively Analyze Impurity Content: Utilize impurity reference standards for quantitative analysis to ensure that the impurity content in pharmaceutical products is within a controllable range and meets quality standards.
Improve Pharmaceutical Quality Control: During pharmaceutical production and research and development, impurity reference standards can serve as important quality control tools to help improve the overall quality of pharmaceutical products.
We are committed to providing high-quality and high-purity Norfloxacin impurity reference standards to meet our customers' needs in drug research and development, quality control, and pharmaceutical testing.




 China
China