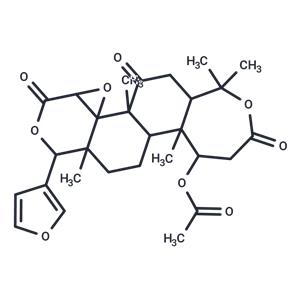
| Name | Nomilin |
| Description | Nomilin is naturally occurring triterpenoids, have immunomodulatory activity. |
| Cell Research | Cell lines: HUVECs. Concentrations: 5 μg-500 μg/ml. Incubation Time: 48 h. Method:. HUVECs were seeded (5000 cells/well) in 96-well flat-bottomed titer plate and incubated for 24 h at 37 °C in 5% CO2 atmosphere. Different concentrations of Nomilin (5 μg-500 μg/ml) were added and incubated further for 48 h. Before 4 h completion of incubation, 20 μl MTT (5 mg/ml) was added. Percentage of dead cells was determined using an ELISA plate reader set to record absorbance at 570 nm. |
| Animal Research | Animal Models: Four to six week old male C57BL/6 mice. Formulation: light paraffin oil. Dosages: 6 mg/kg, I.P. |
| In vitro | Administration of Nomilin significantly retarded endothelial cell proliferation, invasion, migration and tube formation. It also possesses anti-proliferative activity against the number of human cancer cell lines including leukemia (HL-60), ovary (SKOV-3), liver (Hep G2), cervix (HeLa), stomach (NCI-SNU-1), and breast (MCF-7)[2]. Nomilin significantly decreased TRAP-positive multinucleated cell numbers compared with the control and exhibited no cytotoxicity. It decreases bone resorption activity and downregulates osteoclast-specific genes, NFATc1 and TRAP mRNA levels. Furthermore, Nomilin suppressed MAPK signaling pathways. Thus, Nomilin has inhibitory effects on osteoclastic differentiation in vitro[3]. |
| In vivo | Nomilin is an effective inducer of gluthathione S-transferase activity in mice and to inhibit carcinogenesis in the hamster buccal pouch assay. Nomilin can shorten anaesthetic-induced sleeping time in mice, probably through a stimulant action on the central nervous system [1]. Nomilin significantly inhibited tumour-directed capillary formation. Serum proinflammatory cytokines such as IL-1β, IL-6, TNF-α and GM-CSF and also serum NO levels were significantly reduced by the treatment of nomilin. Administration of Nomilin significantly reduced the serum level of VEGF, a proangiogenic factor and increased the antiangiogenic factors TIMP-1 and IL-2 [2]. |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | 10% DMSO+40% PEG300+5% Tween 80+45% Saline : 2 mg/mL (3.89 mM), Sonication is recommended.
H2O : Insoluble
Ethanol : Insoluble
DMSO : 125 mg/mL (242.93 mM), Sonication is recommended.
|
| Keywords | Nomilin | MAPK | limonoid | ischemia-reperfusion | Inhibitor | inhibit | compound | anti-obesity | anti-hyperglycemic |
| Inhibitors Related | Sodium lauryl sulfoacetate | Phosphocreatine disodium hydrate | SKLB-163 | MBM-55S | RV01 | NG25 | RMM-46 | Phosphocreatine disodium | Oleanolic Acid | MK2-IN-3 hydrate | Selonsertib | Sodium new houttuyfonate |
| Related Compound Libraries | Anti-Tumor Natural Product Library | Terpene Natural Product Library | Pain-Related Compound Library | Bioactive Compound Library | Kinase Inhibitor Library | Anti-Inflammatory Traditional Chinese Medicine Compound Library | Natural Product Library | Natural Product Library for HTS | Flavor Natural Product library | Heat-Clearing and Detoxifying Traditional Chinese Medicine Compound Library | Ancient Chinese Classical Formulas Compound Library | Food as Medicine Compound Library |

 United States
United States