N-Nitroso Tadalafil
Product Code: T007086
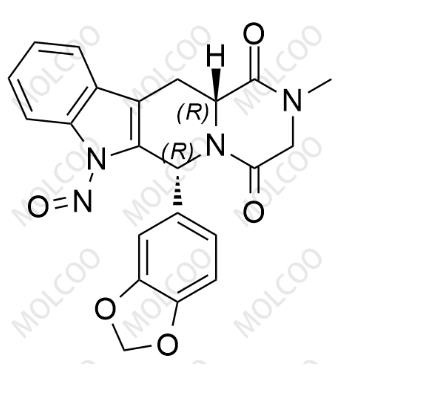
English Name: N-Nitroso Tadalafil
English Alias: (6R,12aR)-6-(benzo[d][1,3]dioxol-5-yl)-2-methyl-7-nitroso-2,3,12,12a-tetrahydropyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4(6H,7H)-dione
CAS No.: Not provided
Molecular Formula: C₂₂H₁₈N₄O₅
Molecular Weight: 418.40
As a nitroso derivative impurity of Tadalafil, it is used for pharmaceutical quality control and safety assessment to ensure that the content of such impurities in Tadalafil API and formulations meets the requirements of pharmacopoeias and regulations.
The nitroso group (-NO) in the structure may serve as a characteristic impurity for impurity profile analysis, helping pharmaceutical companies optimize production processes and reduce potential genotoxic risks.
Drug R&D and Quality Control: As a reference standard for qualitative and quantitative analysis of impurities in Tadalafil-related drugs, such as the development and validation of HPLC, LC-MS and other detection methods.
Safety Evaluation: Nitroso compounds may have potential toxicity. The study of this impurity can assist in evaluating the safety of Tadalafil products and provide data support for drug registration and regulatory submission.
Tadalafil is a PDE5 inhibitor used in the treatment of male erectile dysfunction. During drug production, various impurities may be generated due to synthesis processes, storage conditions, etc. The formation of nitroso impurities (such as N-Nitroso Tadalafil) is related to raw material residues, reaction by-products, or oxidation processes. Nitroso compounds are considered to have potential carcinogenicity in some studies, so pharmacopoeias (such as USP, EP) have become increasingly strict in controlling such impurities, driving the demand and research of related impurity reference standards.
Analytical Method Research: The current detection of nitroso impurities mostly uses high-sensitivity chromatography-mass spectrometry technology, with the focus on optimizing the limit of detection and limit of quantification to meet the regulatory requirements for trace impurities.
Toxicity Risk Assessment: Some studies focus on the potential genotoxicity of such impurities, and establish safe exposure limits through toxicological experiments to provide a basis for pharmaceutical impurity control standards.
Process Control: The pharmaceutical industry is reducing the formation of nitroso impurities by optimizing synthetic routes and improving purification processes. Related impurity reference standards play a key role in process validation.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!