N-Nitroso Ozenoxacin
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Number: N031173
English Name: N-Nitroso Ozenoxacin
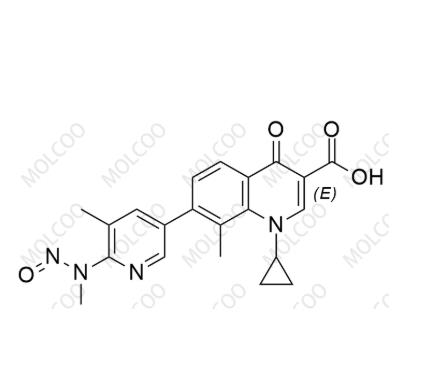
English Alias: 1-cyclopropyl-8-methyl-7-(5-methyl-6-(methyl(nitroso)amino)pyridin-3-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
CAS Number: None
Molecular Formula: C₂₁H₂₀N₄O₄
Molecular Weight: 392.41
As a nitroso derivative impurity of Ozenoxacin, this compound has the following advantages:
Well-defined and distinct structure: Contains nitroso (-N=O) group, quinolinone ring, cyclopropyl, and pyridinyl, differing from Ozenoxacin by methyl(nitroso)amino substitution on pyridine ring. It can be accurately identified by techniques such as HPLC and LC-MS, providing a specific marker for impurity detection;
High stability and traceability: The N-nitrosamine structure is stable under acidic conditions. As a product of amino oxidation during Ozenoxacin storage or degradation, it directly reflects oxidative deterioration, improving quality tracing accuracy;
High detection sensitivity: The conjugated system of quinolinone and nitroso groups shows strong UV absorption (270-290nm), and combined with carboxyl polarity, trace analysis can be achieved via HPLC-UV or LC-MS, compatible with quinolone detection systems.
Pharmaceutical quality control: Used as an impurity reference to identify and quantify N-Nitroso Ozenoxacin in Ozenoxacin APIs and formulations, ensuring residual nitroso impurities meet quality standards;
Stability evaluation: Assessing oxidative degradation trends of Ozenoxacin formulations under varying storage conditions (temperature, humidity) by monitoring impurity levels to support shelf-life determination;
Degradation mechanism studies: Exploring oxidative breakdown pathways of Ozenoxacin, clarifying nitroso impurity formation conditions to optimize antioxidant production processes.
Ozenoxacin is a quinolone antibacterial for skin infections, with a structure containing oxidizable amino groups on the pyridine ring. During storage (especially under air or light exposure), amino groups may oxidize to form nitroso derivatives, namely N-Nitroso Ozenoxacin. Nitrosamines have potential genotoxicity, making their control critical for Ozenoxacin quality assurance and safety evaluation.
Current research focuses on:
Detection optimization: Using UPLC-MS/MS with quinolinone fragment monitoring to achieve ultra-trace detection (ppb level);
Oxidation studies: Investigating impurity formation kinetics under different pH and light conditions to identify key factors driving amino oxidation in Ozenoxacin;
Toxicological assessment: Evaluating genotoxic potential via in vitro tests to establish strict impurity limits;
Stabilization strategies: Developing light-protective packaging and antioxidant formulations to minimize nitroso impurity formation in Ozenoxacin products.
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com