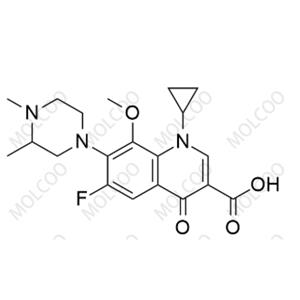
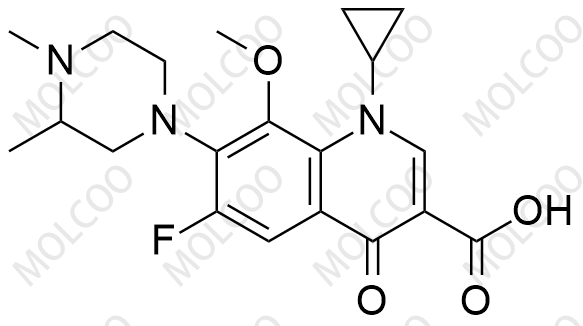
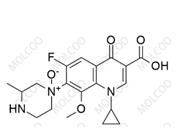
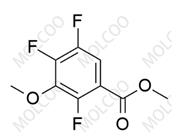
English Name: Gatifloxacin Impurity
CAS Number: Various (e.g., 172426-87-8, 112811-57-1, etc., depending on the impurity type)
Purity Specification: High purity, meeting the needs of scientific research and pharmaceutical manufacturing
Origin: China
Packaging Information: Different packaging specifications are available according to customer requirements
Product Introduction
Gatifloxacin impurity reference standards are crucial chemical substances used in drug development, quality control, pharmacodynamics research, and other fields. Our products offer a variety of gatifloxacin impurities, including but not limited to gatifloxacin des-ethylene impurity, 6-piperazine gatifloxacin (Gatifloxacin USP Impurity D), etc. These impurities are of great significance for assessing the purity, stability, and safety of drugs.
Company Introduction
We are a company specializing in the field of chemical pharmaceuticals, providing high-quality products and services such as complex organic small molecule compounds, impurity reference standards, and API standards. With strong synthetic capabilities and internationally leading scientific research technology, we are committed to providing excellent products and services to customers worldwide.


 China
China