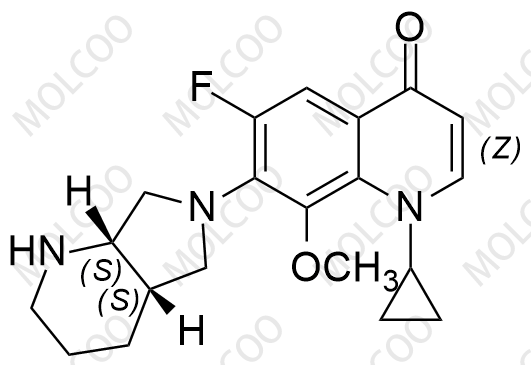
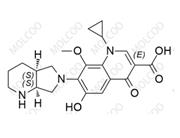
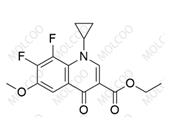
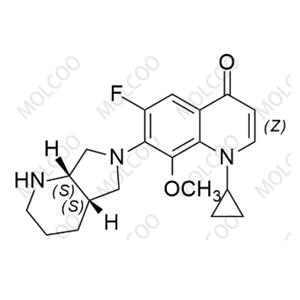
Moxifloxacin Impurity 1322062-57-6
Moxifloxacin Impurity Reference Standards are crucial tools for drug development, production, and quality control. They provide accurate chemical structures and purity standards, ensuring the quality, safety, and effectiveness of Moxifloxacin medications.
We offer a variety of Moxifloxacin Impurity Reference Standards, including but not limited to Impurity A, B, C, D, E, F, I, etc. These impurity reference standards have undergone rigorous synthesis, separation, and purification processes to ensure their purity exceeds 98%, complying with international pharmacopoeias and relevant regulations.
Moxifloxacin Impurity Reference Standards can be used in multiple aspects such as assay determination, thin-layer chromatography identification, infrared spectroscopy identification, and related substances examination. They not only enhance the efficiency of drug development but also provide reliable standards for drug production and quality control.
Our Moxifloxacin Impurity Reference Standards come with detailed structural formulas, CAS numbers, molecular formulas, molecular weights, and other information, facilitating user access and utilization. Additionally, we provide professional technical support and after-sales service to ensure users receive timely assistance and guidance during use.




 China
China