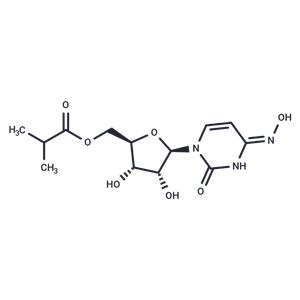
| Name | Molnupiravir |
| Description | Molnupiravir (EIDD-2801) is an isopropyl ester prodrug of the ribonucleoside analog EIDD-1931 with oral bioavailability. Molnupiravir can be used in the study of COVID-19, seasonal and pandemic influenza, and has broad-spectrum antiviral activity against multiple coronaviruses and influenza viruses, such as SARS-CoV-2, MERS-CoV, SARS-CoV. |
| In vitro | METHODS: RD, Huh7, and Vero cells were treated with Molnupiravir (MK-4482) (EIDD-2801) (1, 10, 100 μM) for cytopathic effect (CPE) protection assay.
RESULTS EIDD-2801 showed stable CPE protection potential in a dose-dependent manner in multiple cell lines infected with EV-A71 virus; the value against EV-A71 virus was 70.12±4.40μM in RD cells; 88.52±3.18μM in Vero cells, and 35.64±0.47μM in Huh7 cells. [1] |
| In vivo | METHODS: Molnupiravir (MK-4482) (EIDD-2801) (50, 150, or 500 mg/kg, orally, every 12 hours) was used to prophylactically treat mice infected with SARS-CoV and observe its effects in mice.
RESULTS Molnupiravir has potent antiviral activity and is able to prevent SARS-CoV replication and disease. [1] |
| Storage | store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | DMSO : 40 mg/mL (121.47 mM), Sonication is recommended.
10% DMSO+40% PEG300+5% Tween 80+45% Saline : 2 mg/mL (6.07 mM), Sonication is recommended.
|
| Keywords | SARS-CoV | SARSCoV | Molnupiravir | MK4482 | MK 4482 | InfluenzaVirus | Influenza Virus | Influenza virus | EIDD2801 | EIDD 2801 |
| Inhibitors Related | Acetylcysteine | α-Vitamin E | Silymarin | AEBSF hydrochloride | Hydroxychloroquine | Nitazoxanide | Chloroquine phosphate | Curcumin | Dexamethasone | Naringenin | Salcomine | Crystal Violet |
| Related Compound Libraries | FDA-Approved & Pharmacopeia Drug Library | Failed Clinical Trials Compound Library | Bioactive Compound Library | Approved Drug Library | Drug-induced Liver Injury (DILI) Compound Library | Drug Repurposing Compound Library | Anti-Viral Compound Library | Orally Active Compound Library | Bioactive Compounds Library Max | Anti-Infection Compound Library | Nucleotide Compound Library |

 United States
United States