1. Product information
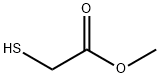
| Product Name: | Methyl thioglycolate |
| Synonyms: | MERCAPTOACETIC ACID METHYL ESTER;METG;METHYL THIOGLYCOLATE;METHYL THIOGLYCOLLATE;METHYL MERCAPTOACETATE;THIOGLYCOLIC ACID METHYL ESTER;mercapto-aceticacimethylester;Methanethiol, methoxy- |
| CAS: | 2365-48-2 |
| MF: | C3H6O2S |
| MW: | 106.14 |
| EINECS: | 219-121-7 |
| Product Categories: | 2365-48-2 |
| Mol File: | 2365-48-2.mol |
 |
|
| Methyl thioglycolate Chemical Properties |
| Melting point | -24 °C |
| Boiling point | 42-43 °C/10 mmHg (lit.) |
| density | 1.187 g/mL at 25 °C (lit.) |
| refractive index | n20/D 1.466(lit.) |
| Fp | 86 °F |
| storage temp. | Store below +30°C. |
| solubility | 40g/l |
| form | Liquid |
| pka | 8.04±0.10(Predicted) |
| color | Clear colorless |
| Water Solubility | 40 g/L (20 ºC) |
| Chemical Properties | CLEAR COLOURLESS LIQUID |
| Uses | Methyl Thioglycolate is an derivative of Thioglycolic Acid (T350760), an organic compound containing both a thiol and a carboxylic acid functional groups. Thioglycolic Acid and its derivatives are oft en used in organic synthesis as a nucleophile in thioglycolysis reactions and is also used as a S transfer agent for sulfonyl chloride synthesis. Methyl Thioglycolate have been studied for the its inf luence on neocarzinostatin activation and expression of DNA damage. |
| Uses | Methyl thioglycolate was used in the preparation of 3-carbomethoxy-4- oxotetrahydrothiopyran, 2- and 4-carbomethoxy-3-oxotetrahydrothiophene. It is used to prepare methyl thioglycolate and aminoethanethiol conjugated gold nanorods. |
| Application | Methyl thioglycolate is a chemical compound that is used in the pharmaceutical industry as an intermediate. It has been used to synthesize anti-inflammatory drugs, such as aspirin and ibuprofen, and also for the treatment of autoimmune diseases. Methyl thioglycolate binds to amino acids on the surface of bacteria, which prevents them from functioning properly. This binding causes the cells to stop growing and die. |
| Definition | Methyl thioglycolate are widely used in free-radical photoinitiated polymerization reactions. In particular, the use of methyl thioglycolate (MTG), CH3OC(O)CH2SH, results in greater photopolymerization reaction rates, attributed to a weakening of the sulfur–hydrogen bond by hydrogen bonding of the thiol group. MTG constitutes the smaller exponent of the series of CH3OC(O)(CH2)nSH compounds, reported as intermediated in the organic sulfur cycle in marine environments, produced by marine phytoplankton. |
2. Packaging
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping

4. Contact information
For more details, pls contact us freely.
Email address : elin@fdachem.com
Mob: 86 13613820652
WhatsApp/Skype/Wechat/LINE: 86 13613820652


 China
China






