| CAS: | 598-55-0 |
| MF: | C2H5NO2 |
| MW: | 75.07 |
| EINECS: | 209-939-2 |
| Product Categories: |
|
| Mol File: | 598-55-0.mol |
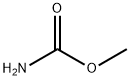 |
|
| Methyl carbamate Chemical Properties |
| Melting point | 56-58 °C (lit.) |
| Boiling point | 176-177 °C (lit.) |
| density | 1,14 g/cm3 |
| refractive index | 1.4125 |
| Fp | 93 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | 700 g/L (20°C) |
| pka | 13.37±0.50(Predicted) |
| form | Adhering Crystals |
| color | White |
| PH | 6-8 (50g/l, H2O, 20℃) |
| Water Solubility | 700 g/L (20 ºC) |
| Sensitive | Moisture Sensitive |
| Merck | 14,6036 |
| BRN | 635779 |
| CAS DataBase Reference | 598-55-0(CAS DataBase Reference) |
| IARC | 3 (Vol. 12, Sup 7) 1987 |
| NIST Chemistry Reference | Carbamic acid, methyl ester(598-55-0) |
| EPA Substance Registry System | Methyl carbamate (598-55-0) |
| Methyl carbamate Usage And Synthesis |
| Chemical Properties | WHITE ADHERING CRYSTALS |
| Uses | Methyl carbamate was used in the synthesis of protected aminocyclopropanes. |
| Preparation | Methyl carbamate was synthesized by the following preparation. MDI (5.00 g,0.020mol) and 100 ml of dried acetone were weighed into a Ng-filled three-neck flask.Methanol (5 g, an excess amount) is added dropwise to the stirred MDI solution in the flask,and then the solution was heated to 56 C. After 24 hrs, the reaction mixture was cooled down to room temperature and evaporated to give a crude solid at about 100% yield. The following recrystallization procedure gave 3.1 g of a white solid (0.012 mol,60% yield). The disappearance of FT-IR stretching band of the isocyanate group (-N=C=O, 2280 cm') confirmed that the reaction was complete. |
| Definition | ChEBI: Methyl carbamate is a carbamate ester resulting from the formal condensation of the carboxy group of carbamic acid with methanol. |
| General Description | White crystals. |
| Air & Water Reactions | Water soluble. |
| Reactivity Profile | Methyl carbamate is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates. |
| Fire Hazard | Flash point data for Methyl carbamate are not available; however, Methyl carbamate is probably combustible. |
| Safety Profile | Poison by ingestion and intraperitoneal routes. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. See also CARBAMATES. |
| Purification Methods | Crystallise the carbamate from *benzene or distil it. [Beilstein 3 H 21.] |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
