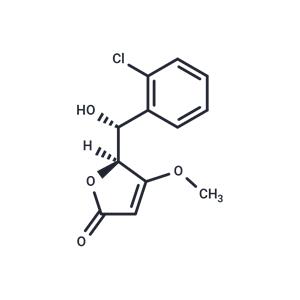
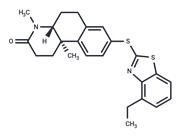
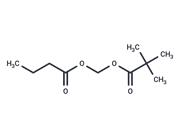
| In vivo | Losigamone (AO-33) was tested in 52 healthy male volunteers in 4 placebo-controlled phase I studies. In the study 1 single dose of 100, 200, 300, 500, 700, and 1,000 mg losigamone was given as a fast-releasing capsule to 12 subjects. The pharmacokinetics of losigamone measured after administration of 100, 300, and 700 mg was linear. Clearance and t1/2 were about 350 ml/min and 4 h, respectively, the Cmax values of 0.7, 1.7, and 4.4 micrograms/ml were reached after 2.5 h. In study, 2,500 mg losigamone was given as a fast-release capsule for 6 days (t.i.d.) to 12 subjects. There was a small but statistically significant decrease for the AUC but no change in t1/2, Cmax, or tmax comparing single dose kinetics on days 1 and 8. There appeared to be no change in caffeine clearance on days 1 and 9. Study 2 was repeated in 20 volunteers with a film-coated tablet. Pharmacokinetic parameters appeared to be unaffected by this change in galenical formulation. In the study 4 daily doses of 400, 1,200, and 1,800 mg losigamone were given 28 days to 24 subjects. The kinetics of caffeine and antipyrine were compared on days 1 and 29. With the exception of t1/2 for antipyrine in the 400 mg group there was no statistically significant change in pharmacokinetic parameters. Generally, losigamone was well tolerated and no serious adverse side effects occurred. In some subjects, a reversible increase in transaminases was observed.[1] |

 United States
United States