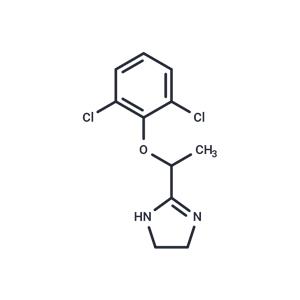
| Name | Lofexidine |
| Description | Lofexidine, a selective α2-receptor agonist, reduces narcotic withdrawal symptoms. |
| Animal Research | 8-day, randomized, double-blind, placebo-controlled, parallel-group study in 264 patients dependent on short-acting opioids evaluated the efficacy of lofexidine hydrochloride in reducing withdrawal symptoms in patients undergoing opioid withdrawal.?The primary efficacy measures were SOWS-Gossop on Day 3 and time-to-dropout.?Secondary endpoints included the proportion of participants who were completers;?area under the 5-day SOWS-Gossop - time curve (i.e., AUC1-5), and daily mean SOWS-Gossop, OOWS-Handelsman, MCGI (subject and rater), and VAS-E scores.?Participants received lofexidine HCl 3.2mg daily in four divided doses or matching placebo on Days 1-5, followed by 2days of placebo[1]. |
| In vivo | Lofexidine significantly decreased SOWS scores compared to placebo and demonstrated better retention rates in participants undergoing opioid withdrawal. Lofexidine potentially offers a useful non-opioid alternative to treat opioid withdrawal symptoms[1]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 62.5 mg/mL (241.19 mM)
|
| Keywords | inhibit | Inhibitor | Lofexidine | Beta Receptor | Adrenergic Receptor |
| Inhibitors Related | Olanzapine | Mirtazapine | Octopamine hydrochloride | Gemfibrozil | Dexmedetomidine hydrochloride | Phenylephrine hydrochloride | Isoprenaline hydrochloride | Amitriptyline hydrochloride | Trazodone hydrochloride | Mianserin hydrochloride |
| Related Compound Libraries | Bioactive Compound Library | Anti-Neurodegenerative Disease Compound Library | Membrane Protein-targeted Compound Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Anti-Cancer Approved Drug Library | FDA-Approved Drug Library | Bioactive Compounds Library Max | GPCR Compound Library | Anti-Cancer Drug Library |

 United States
United States