| CAS: | 38721-52-7 |
| MF: | C12H28B.Li |
| MW: | 190.1 |
| EINECS: | 254-101-1 |
| Product Categories: | B (Classes of Boron Compounds);Classes of Metal Compounds;Li (Lithium) Compounds;Reduction;Synthetic Organic Chemistry;Tetrahydroborates;Typical Metal Compounds;38721-52-7 |
| Mol File: | 38721-52-7.mol |
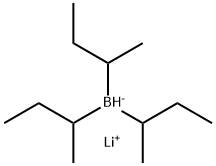 |
|
| Lithium triisobutylhydroborate Chemical Properties |
| density | 0.89 g/mL at 25 °C |
| Fp | 1 °F |
| storage temp. | Flammables area |
| solubility | Miscible with tetrahydrofuran. |
| form | Liquid |
| color | Colorless to yellow |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| BRN | 4007327 |
| InChIKey | ACJKNTZKEFMEAK-UHFFFAOYSA-N |
| CAS DataBase Reference | 38721-52-7(CAS DataBase Reference) |
| EPA Substance Registry System | Borate(1-), hydrotris(1-methylpropyl)-, lithium, (T-4)- (38721-52-7) |
| Lithium triisobutylhydroborate Usage And Synthesis |
| Description | Lithium tri-sec-butylborohydride, commercially available as a one molar solution in tetrahydrofuran. It is widely used as a mild and strong reducing agent with high stereoselectivity in organic synthesis. |
| Features | Lithium tri-sec-butylborohydride is a new type of hydride reducing agent, which has the characteristics of strong reducing ability and high regio- and stereoselectivity. |
| Preparation | Lithium triisobutylhydroborate can be prepared by:In a 3000ml three-necked flask, add 1000ml of 1mol/L tri-sec-butylboron tetrahydrofuran solution, add 1000ml of 1mol/L triethylenediamine tetrahydrofuran solution simultaneously, introduce nitrogen to start stirring, put the mixture of the two in an ice-water bath After cooling to 0° C., 1000 ml of a 1 mol/L lithium aluminum hydride solution in tetrahydrofuran was slowly added dropwise to the above mixed solution with a constant pressure funnel for about 10 hours. |
| Physical properties | clear colourless solution |
| Uses | Lithium triisobutylhydroborate is used as a reducing agent. Reagent for: Enantioselective synthesis of a-amino acids via reduction of N-tert-butanesulfinyl ketimine esters. Diastereoselective reduction reactions. Hydride reduction of the Danishefsky pyranones. Asymmetric reductive aldol reaction of enones with a-alkoxy aldehydes. Synthesis of alkanols by carbonylation reactions. |
| Uses | L-Selectride Solution is a reducing agent which is used in the preparation of oxygen heterocycles. Used also as a reactant in the synthesis of novel bicicyclo[3.1.0]hexane analags which may prove useful in the treatment of depression. |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
Advantage
In stock










 China
China

 Company information
Company information


