1/5
Lithium nitrate NEW
- Min. Order1KG
- Purity99%
- Cas No7790-69-4
- Supply Ability50000KG/month
- Update time2025-03-21

| Product Name | Lithium nitrate |
| CAS No | 7790-69-4 |
| EC-No | 232-218-9 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2025/03/21 |
| CAS: | 7790-69-4 |
| MF: | LiNO3 |
| MW: | 68.95 |
| EINECS: | 232-218-9 |
| Product Categories: | metal nitrate salts;Essential Chemicals;Reagent Plus;Routine Reagents;ICP CRMsAnalytical Standards;ICP-OES/-MS;AAS;AAS CRMsAnalytical Standards;AASSpectroscopy;Alphabetic;Application CRMs;L;Spectroscopy;ICPSpectroscopy;Inorganics;LITHIUM COMPOUNDS;Inorganic Salts;Lithium;Synthetic Reagents |
| Mol File: | 7790-69-4.mol |
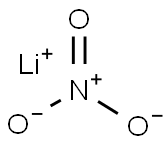 | |
| Lithium nitrate Chemical Properties |
| Melting point | 264 °C (lit.) |
| Boiling point | 600 °C |
| density | 2.38 |
| vapor pressure | 0Pa at 25℃ |
| RTECS | QU9330000 |
| Fp | 600°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| pka | 2.34[at 20 ℃] |
| form | Solid |
| color | White |
| Specific Gravity | 2.38 |
| PH | 7-9 (50g/l, H2O, 20℃) |
| Water Solubility | 90 g/L (28 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,5536 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Stability: | Stable. Incompatible with reducing agents, strong acids, organic materials, finely powdered metals. Oxidizer. |
| CAS DataBase Reference | 7790-69-4(CAS DataBase Reference) |
| EPA Substance Registry System | Lithium nitrate (7790-69-4) |
| Lithium nitrate Usage And Synthesis |
| Chemical properties | Lithium Nitrate is an inorganic compound with the formula LiNO3 with highly water solubility. All metallic nitrates are inorganic salts of a given metal cation and the nitrate anion. The nitrate anion is a univalent (-1 charge) polyatomic ion composed of a single nitrogen atom ionically bound to three oxygen atoms for a total formula weight of 62.05. Nitrate compounds are generally soluble in water. Lithium Nitrate is white trigonal crystal. It is soluble in water, ethanol, pyridine and ammonia. The aqueous solution is neutral.  |
| Solubility in water (g/100ml) | Dissolved amount (g) per 100 milliliters of water at different temperatures (° C) 53.4g/0℃;60.8g/10℃;70.1g/20℃;138g/30℃;152g/40℃;175g/60℃ |
| Toxicity |
|
| Application |
|
| Preparation | Lithium hydroxide or lithium carbonate is added to the reactor containing distilled water, and nitric acid is slowly added under stirring and heating to carry out the reaction. Then we evaporate it to dryness and heat it under vacuum at about 200 ° C, finally we get lithium nitrate. HNO3+LiOH→LiNO3+H2O |
| Explosive characteristics | May cause the acceleration of the burning of combustible materials. Prolonged exposure to heat or flames may result in an explosion. |
| References | https://pubchem.ncbi.nlm.nih.gov/compound/Lithium_nitrate#section=Top https://www.americanelements.com/lithium-nitrate-7790-69-4 https://en.wikipedia.org/wiki/Lithium_nitrate |
| Chemical Properties | Lithium nitrate is a colorless deliquescent powder, enthalpy of fusion 24.90 kJ/mol; can be prepared by reaction of HNO3 with LiOH or Li2CO3, followed by evaporation to dryness and then heating at ~200°C in vacuum; used in ceramics, pyrotechnics, molten salt baths, rocket propellants, refrigerators. [HAW93] [CRC10] [MER06] [KIR81] [FMC93] |
| Uses | Lithium nitrate can be used in special pyrotechnic devices to give a red flame. Lithium nitrate may also be used in combination with other salts, particularly nitrates, to produce low-melting fused salt mixtures. Used in ceramics, heat-exchange media, refrigeration systems. |
| Uses | Lithium nitrate is used in ceramics, pyrotechnics, salt baths, heat-exchange media, refrigeration systems, rocket propellant and in solar batteries. It acts as an oxidizing agent used in the production of red-colored fireworks and flares, as a catalyst to accelerate the breakdown of nitrogen oxides and as a medium to store heat received from sun for cooking. It is applied to concrete-pavement to withstand weathering effects. |
| Definition | ChEBI: The inorganic nitrate salt of lithium. |
| Preparation | Lithium nitrate is readily prepared industrially or in the laboratory by the reaction of lithium hydroxide or lithium carbonate with nitric acid. The resulting solution is evaporated and the solid is dried to obtain anhydrous lithium nitrate. Lithium nitrate is very soluble in water. The compound absorbs moisture from the air. Below about 30 °C it crystallizes from solution as lithium nitrate trihydrate; above that temperature the anhydrous material precipitates although the question of the existence of a half-hydrate is not entirely settled. |
| General Description | A white to light yellow colored crystalline solid. Denser than water. Contact with the material may cause irritation to skin, eyes, and mucous membranes. May be toxic by ingestion. May cause the acceleration of the burning of combustible materials. Prolonged exposure to heat or flames may result in an explosion. |
| Air & Water Reactions | Deliquescent. Soluble in water. |
| Reactivity Profile | Lithium nitrate is an oxidizing agent. Mixtures with alkyl esters may explode owing to the formation of alkyl nitrates; mixtures with phosphorus, tin(II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109]. |
| Health Hazard | Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. |
| Fire Hazard | These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard. |
| Flammability and Explosibility | Nonflammable |
| Potential Exposure | Lithium nitrate is used in ceramics, pyrotechnics, salt baths; refrigeration systems; and rocket propellants |
| Shipping | UN2722 Lithium nitrate, Hazard Class: 5.1; Labels: 5.1-Oxidizer |
| Purification Methods | It crystallises from water or EtOH. Dry it at 180o for several days by repeated melting under vacuum. If it is crystallised from water keeping the temperature above 70o, formation of trihydrate is avoided. The anhydrous salt is dried at 120o and stored in a vacuum desiccator over CaSO4. After the 99% pure salt was recrystallised 3times, it contained: metal (ppm) Ca (1.6), K (1.1), Mo (0.4), Na (2.2). [Donnan & Burt J Chem Soc 83 335 1903.] |
| Incompatibilities | May explode when exposed to sparks, shock and heat. Violent reactions with combustible materials; oxidizers, organic materials; reducing agents; strong acids |
Product picture

Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
-







