1. Product information
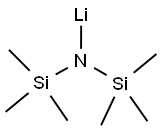
| Product Name: | Lithium bis(trimethylsilyl)amide |
| Synonyms: | 1,1,1,3,3,3-HEXAMETHYLDISILAZAN LITHIUMSALZ;1,1,1,3,3,3-HEXAMETHYLDISILAZANE LITHIUM SALT;HEXAMETHYLDISILAZANE LITHIUM SALT;LHMDS;LIHMDS;LITHIUM HEXAMETHYLDISILAZANE;LITHIUM HEXAMETHYLDISILAZIDE;LITHIUM-BIS-(TRIMETHYLSILYL)-AMID |
| CAS: | 4039-32-1 |
| MF: | C6H18LiNSi2 |
| MW: | 167.33 |
| EINECS: | 223-725-6 |
| Product Categories: | Classes of Metal Compounds;Li (Lithium) Compounds;Si (Classes of Silicon Compounds);Silazanes;Silicon Compounds (for Synthesis);Si-N Compounds;Synthetic Organic Chemistry;Typical Metal Compounds |
| Mol File: | 4039-32-1.mol |
 |
|
| Lithium bis(trimethylsilyl)amide Chemical Properties |
| Melting point | 73°C |
| Boiling point | 55-56 °C |
| density | 0.860 g/mL at 25 °C (lit.) |
| refractive index | n20/D 1.425(lit.) |
| Fp | 48 °F |
| storage temp. | Store below +30°C. |
| solubility | hydrolysis |
| form | Powder |
| color | White |
| Specific Gravity | 0.716 |
| Water Solubility | hydrolysis |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 3567910 |
| Exposure limits | ACGIH: TWA 50 ppm; STEL 100 ppm (Skin)
OSHA: TWA 200 ppm(590 mg/m3)
NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) |
| InChIKey | YNESATAKKCNGOF-UHFFFAOYSA-N |
| CAS DataBase Reference | 4039-32-1(CAS DataBase Reference) |
| EPA Substance Registry System | Silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, lithium salt (4039-32-1) |
| Hazard Codes | F,C,N |
| Risk Statements | 11-34-48/20-63-65-67-51/53-35-20-62-14-40-37-19 |
| Safety Statements | 9-16-26-29-33-36/37/39-45-61-62-57-43 |
| RIDADR | UN 2925 4.1/PG 2 |
| WGK Germany | 3 |
| F | 10-23 |
| TSCA | Yes |
| HazardClass | 4.3 |
| PackingGroup | II |
| HS Code | 29319090 |
|
| Lithium bis(trimethylsilyl)amide Usage And Synthesis |
| Chemical Properties | light yellow to yellow crystalline powder |
| Physical properties | distillable low-melting solid; mp 70–72 °C, bp 115 °C/1mmHg. LHMDS is a cyclic trimer in the solid state,3 whereas in benzene solution it exists in a monomer–dimer equilibrium. LHMDS exists as a tetramer-dimer mixture in hydrocarbons and as a dimer-monomer mixture in THF and ether. Treatment of LHMDS with trialkylamines increases the monomer concentration, whereas the use of diamines affords exclusively the corresponding chelated monomer. LHMDS is less soluble, less basic, more stable, and much less sensitive to air compared to lithium diisopropylamide. pKa 29.5 (THF, 27 °C). |
| Uses | Lithium bis(trimethylsilyl)amide is used as nonnucleophilic base to generate kinetic ketone and ester enolates. It is considerably more selective than LDA and undesired reductions (e.g., of nonenolizable ketones observed in the case of LDA) can be avoided by using LHMDS. |
| Uses | Lithium Hexamethyldisilazide is widely used as strong nonnucleophilic base |
| Uses | Lithium bis(trimethylsilyl)amide is a base used in preparation of dienes and enolates. It is used to catalyze the addition of phosphine P-H bonds to carbodiimides leading to phosphaguanidines. Lithium bis(trimethylsilyl)amide is also used in a novel three-step synthesis of disubstituted 1,2,5-thiadiazoles. |
| Preparation | It is conveniently prepared by the reaction of hexamethyldisilazane with n-butyllithium in hexane. For most uses the hexane is then evaporated and replaced with THF. |
| General Description | This product, 0.5 M in 2-methyltetrahydrofuran aligns with Safer Solvents and Auxiliaries, Use of Renewable Feedstocks and Inherently Safer Chemistry for Accident Prevention. |
| Flammability and Explosibility | Highly flammable |
2. Packaging
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping

4. Contact information
For more details, pls contact us freely.
Email address : elin@fdachem.com
Mob: 86 13613820652
WhatsApp/Skype/Wechat/LINE: 86 13613820652

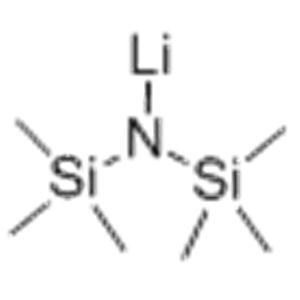

 China
China