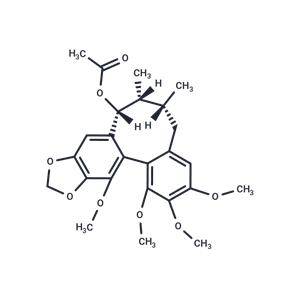
| Name | Kadsurin |
| Description | Kadsurin has anti-oxidant activity, it induces enzymes capable of scavenging oxygen radical species in the liver. Kadsurin may be valuable antitumor promoters or chemopreventors. |
| In vitro | Two new lignans, interiotherins C (1) and D (2), together with the known compounds interiorin (3), heteroclitin F (4), neokadsuranin (5), heteroclitin D (6), Kadsurin (7), gomisin A (8), schisandrin C (9), interiotherin A (10), angeloylgomisin R (11), gomisin G (12), interiotherin B (13), and gomisin C (14), were isolated from the stems of Kadsura interior. The structures and stereochemistries of the new compounds were determined from mass, CD, and NMR spectral data. Fourteen neolignans were screened as potential antitumor promoters by examining their ability to inhibit Epstein-Barr virus early antigen (EBV-EA) activation (induced by 12-O-tetradecanoylphorbol-13-acetate) in Raji cells. Neokadsuranin (5) and schisandrin C (9) were the most potent compounds. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 11 mg/mL (23.99 mM)
|
| Keywords | Kadsurin | Inhibitor | inhibit |
| Inhibitors Related | Butylated hydroxytoluene | Hydroquinone diacetate | Octyl gallate | Methyl 3,4-dihydroxybenzoate | Butylhydroxyanisole | Oxothiazolidinecarboxylic acid | Neohesperidin | Sulbutiamine | (-)-Fenchone | m-Coumaric acid | N,N'-Dimethylthiourea | Trolox |
| Related Compound Libraries | Anti-Tumor Natural Product Library | Bioactive Compound Library | Traditional Chinese Medicine Monomer Library | Selected Plant-Sourced Compound Library | Natural Product Library | Anti-Fibrosis Compound Library | Natural Product Library for HTS | Anti-Aging Compound Library | Bioactive Compounds Library Max | Anti-Cancer Active Compound Library |

 United States
United States