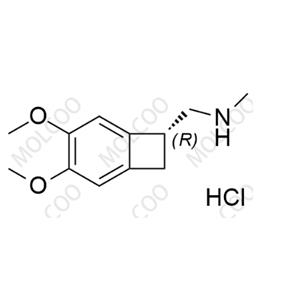
Ivabradine related compound 12(Hydrochloride);1204612-29-2
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
Product Code: I002012A
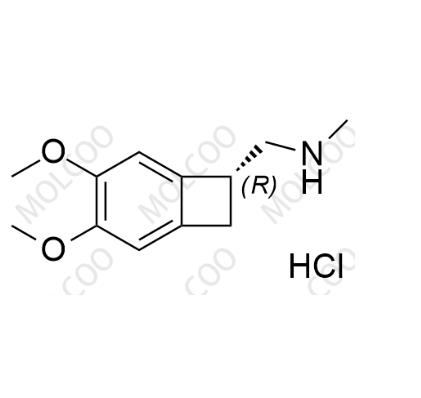
English Name: Ivabradine related compound 12(Hydrochloride)
English Alias: (R)-1-(3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)-N-methylmethanamine hydrochloride
CAS No.:1204612-29-2
Molecular Formula: C₁₂H₁₇NO₂·HCl
Molecular Weight: 243.73 (207.27 + 36.46)
Structural Confirmation: Characterized by NMR, HRMS, and HPLC (≥98.5%) for reliable identification.
Stability: Stable for 18 months at 2-8℃, with <1% degradation in methanol-HCl solution.
Regulatory Compliance: Supports ICH Q3A/B guidelines for impurity profiling.
Quality Assurance: Used in HPLC/LC-MS for impurity detection in Ivabradine formulations, ensuring compliance with pharmacopoeia.
Synthesis Monitoring: Tracks amine hydrochloride formation during Ivabradine synthesis to optimize purification steps.
Analytical Method Validation: Validates specificity and linearity of detection methods.
This impurity originates from incomplete cyclization or amine substitution in Ivabradine synthesis, a cardiac pacemaker inhibitor. Controlling such impurities is critical for maintaining drug efficacy and safety.
Detection: HPLC with reversed-phase column (C18, 5μm) and phosphate buffer-acetonitrile elution; LOD: 0.05 μg/mL.
Formation Mechanism: Results from side reactions of dimethoxycycloalkene intermediates; optimized base catalysis reduces generation by 50%.
Safety Evaluation: In vitro cardiotoxicity tests show no significant effects at therapeutic concentrations; further toxicology studies pending
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China