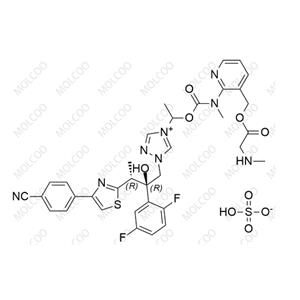
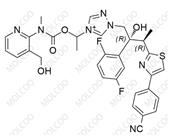
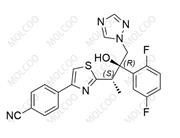
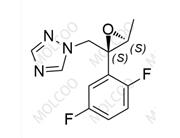
Isavuconazole Sulfate 946075-13-4
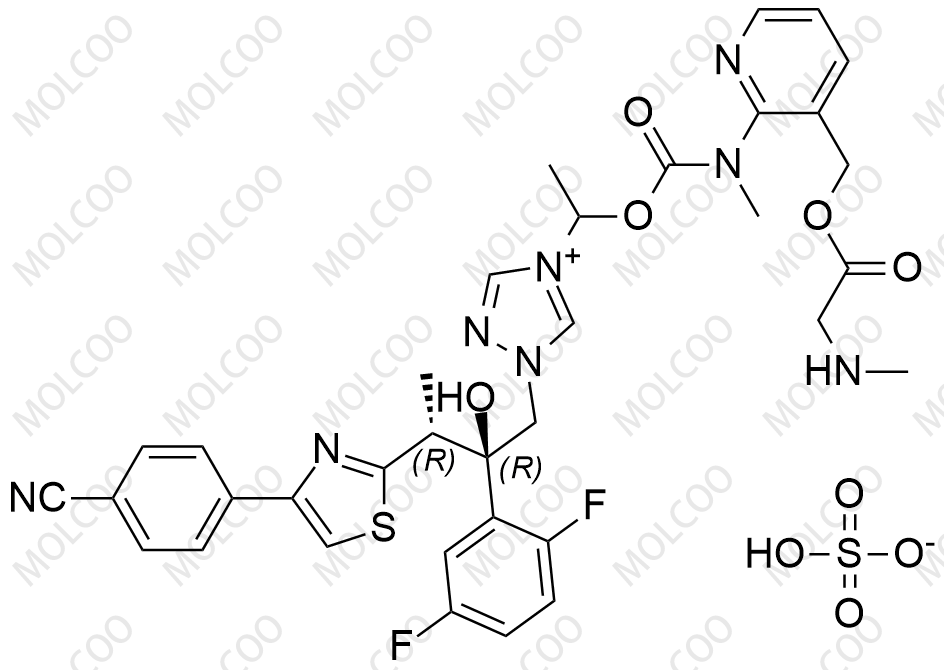

Product Name: Isavuconazole Impurity Reference Standard
Product Details:
Isavuconazole impurity reference standards are high-purity reference materials used in drug development, quality control, and pharmaceutical analysis. These impurity reference standards assist researchers and quality control experts in accurately identifying and quantifying various impurities that may be present in isavuconazole drugs, thereby ensuring their safety and effectiveness.
Main Components:
This product primarily contains multiple specific impurities of isavuconazole, which are carefully extracted from isavuconazole active pharmaceutical ingredients (APIs) or related formulations using advanced separation and purification techniques.
Product Characteristics:
High Purity: Rigorous purification processes ensure that the impurities meet analytical purity requirements.
Excellent Stability: Under appropriate storage conditions, the impurity reference standards remain stable and are resistant to degradation or deterioration.
Easy to Use: The impurity reference standards are typically provided in solid form, facilitating weighing and dissolution, and are compatible with various analytical instruments and methods.
Applications:
Drug Development: Used to identify and quantify potential impurities during the synthesis and formulation development of isavuconazole drugs, ensuring drug quality.
Quality Control: Monitored and controlled the impurity content during the production of isavuconazole pharmaceuticals, ensuring compliance with quality standards.
Pharmaceutical Analysis: Served as reference materials for calibrating instruments and analytical methods during drug testing and inspection processes, improving the accuracy and reliability of analysis.
Storage Conditions:
Store according to the instructions on the packaging, typically in a dry, light-protected, and cool place, to ensure the stability and long-term availability of the impurity reference standards.

 China
China