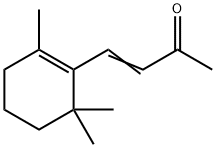
| Description | β-Ionone has a characteristic violet-like odor, more fruity and woody than α-ionone. May be prepared by condensing citral with acetone to form pseudoionone, which is then cyclized by acid-type reagents. |
| Chemical Properties | β-Ionone has a characteristic violet-like odor, more fruity and woody than α-ionone |
| Chemical Properties | clear slightly yellow to yellow liquid |
| Occurrence | Reported found in the distillate from flowers of Boronia megatisma Nees. Also reported found in apricot, orange juice, lemon peel oil, guava, grapes, melon, papaya, peach, raspberry, blackberry, carrot, peas, bell pepper, tomato, ginger, peppermint and spearmint oil, milk powder, beef, hop oil, cognac, whiskies, grape wines, tea, passion fruit, plum, beans, mushroom, starfruit, almonds, mango, fenugreek, tamarind, apple and pear brandy, rice bran, quince, prickly pear, sweet potato, buckwheat, corn oil, corn tortillas, loquat, mountain papaya, clary sage and other sources |
| Uses | beta-Lonone is an essential oil extract from the leaves and flowers of Succisa Pratensis that shows antioxidant and antimicrobial activities against bacteria. |
| Uses | β-Ionone may be used as a reference standard in the determination of β-ionone in wine using solid-phase extraction followed by gas chromatography with mass spectrometry (GC-MS). |
| Definition | ChEBI: Beta-ionone is an ionone that is but-3-en-2-one substituted by a 2,6,6-trimethylcyclohex-1-en-1-yl group at position 4. It has a role as an antioxidant and a fragrance. |
| Preparation | By condensing citral with acetone to form pseudoionone, which is then cyclized by acid-type reagents. |
| Aroma threshold values | Detection: 0.007 to 205 ppb |
| Taste threshold values | Taste characteristics art 10 ppm: woody, sweet, fruity, berry-like with a green berry background |
| General Description | Colorless to light yellow liquid with an odor of cedar wood. In very dilute alcoholic solution the odor resembles odor of violets. Used in perfumery. |
| Air & Water Reactions | Slightly water soluble . |
| Reactivity Profile | Irisone may react vigorously with oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. |
| Health Hazard | ACUTE/CHRONIC HAZARDS: Toxic. May cause allergic reaction. |
| Flammability and Explosibility | Nonflammable |
| Biochem/physiol Actions | Taste at 1-20 ppm |

 Japan
Japan







 Company information
Company information Advantage
Advantage


