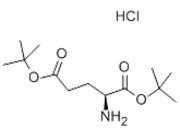
Peptide Synthesis: H-Glu(OtBu)-NH2·HCl serves as a building block in solid-phase peptide synthesis. The t-butoxycarbonyl (t-Bu) protecting group allows for selective deprotection of the amino acid residue during the synthesis process, enabling coupling with other amino acids. This amino acid derivative is particularly useful for the synthesis of peptides containing glutamic acid residues.
Biochemical Studies: H-Glu(OtBu)-NH2·HCl can be used as a substrate or inhibitor in biochemical assays to study the enzymatic activity of proteases, peptidases, or other enzymes that cleave or modify peptides. The presence of the t-Bu protecting group ensures stability during the assay conditions, allowing for accurate measurements. Drug Discovery: In the field of drug discovery, H-Glu(OtBu)-NH2·HCl can be used as a starting material for the synthesis of peptide-based drugs or drug candidates. The glutamic acid residue, being a naturally occurring amino acid, often confers desirable biological properties, such as binding affinity to receptors or modulation of cellular processes. Bioconjugation and Biomaterials: The amine functionality of H-Glu(OtBu)-NH2·HCl allows for conjugation with other molecules or surfaces, enabling the preparation of bioconjugates or the immobilization of peptides on biomaterials. This can be useful for biosensor development, drug delivery systems, or tissue engineering applications. | 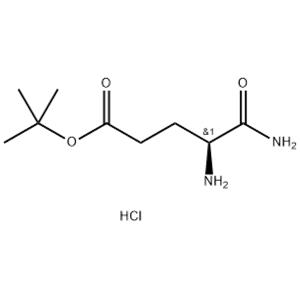

 China
China