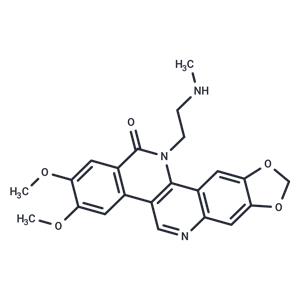
| Name | Genz-644282 |
| Description | Genz-644282, a novel non-camptothecin topoisomerase I inhibitor for cancer treatment. |
| Cell Research | Colony-forming assays were conducted with mouse and human bone marrow and eight human tumor cell lines. In addition, 29 human tumor cell lines representing a range of histology and potential resistance mechanisms were assayed for sensitivity to Genz-644282 in a 72-hour exposure assay. The efficacy of Genz-644282 was compared with standard anticancer drugs (i.e., irinotecan, docetaxel, and dacarbazine) in human tumor xenografts of colon cancer, renal cell carcinoma, non-small cell lung cancer, and melanoma[1]. |
| Animal Research | Genz-644282 was tested against the PPTP in vitro panel (0.1 nM to 1 μM), and in vivo using three times per week × 2 schedule repeated at day 21 at its maximum tolerated dose (MTD) of 4 mg/kg. Subsequently Genz-644282 was tested at 4, 3, 2, and 1 mg/kg in 3 models to assess the dose-response relationship. mRNA gene signatures predictive for Genz-644282 response in vitro were applied to select 15 tumor models that were evaluated prospectively[2]. |
| In vitro | Human bone marrow CFU-GM was more sensitive to the Top1 inhibitors than was mouse bone marrow CFU-GM. The ratio of mouse to human IC(90) values was more than 10 for the camptothecins and less than 10 for Genz-644282, which had more potency as a cytotoxic agent toward human tumor cells in culture than the camptothecins in the colony-forming and 72-hour proliferation assays. Genz-644282 has superior or equal antitumor activity in the human tumor xenografts than the standard drug comparators[1]. |
| In vivo | In vivo, Genz-644282 at its MTD (4 mg/kg) induced maintained complete responses (MCR) in 6/6 evaluable solid tumor models. At 2 mg/kg Genz-644282 induced CR or MCR in 3/3 tumor models relatively insensitive to topotecan, but there were no objective responses at 1 mg/kg. Further testing at 2 mg/kg showed that Genz-644282 induced objective regressions in 7 of 17 (41%) models. There was a significant correlation between predictive response scores based on Affymetrix U133Plus2 baseline tumor expression profiles and the observed in vivo responses to Genz-644282[2]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 5 mg/mL (12.27 mM)
|
| Keywords | Genz 644282 | Genz-644282 | Inhibitor | inhibit | Topoisomerase |
| Inhibitors Related | Berberine chloride | EIDD-1931 | Flumequine | Norfloxacin | Prulifloxacin | Enoxacin | Pefloxacin Mesylate | Camptothecin | Ciprofloxacin | Ciprofloxacin monohydrochloride | Irinotecan hydrochloride trihydrate | Etoposide |
| Related Compound Libraries | DNA Damage & Repair Compound Library | Bioactive Compound Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Inhibitor Library | NO PAINS Compound Library | Anti-Aging Compound Library | Bioactive Compounds Library Max | Anti-Cancer Drug Library | Anti-Cancer Active Compound Library |

 United States
United States