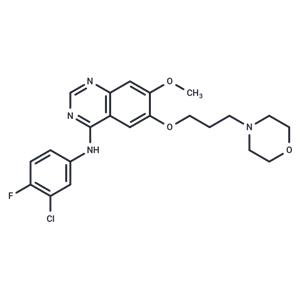
| Name | Gefitinib |
| Description | Gefitinib (ZD1839) is an EGFR first-generation inhibitor with oral activity that inhibits the EGFR 19 Del and L858R mutations. Gefitinib has antitumor activity and is used for the treatment of EGFR-mutated non-small-cell lung cancers. Gefitinib administration RESULTS in the development of the EGFR C797S resistance mutation. |
| Cell Research | The human NSCLC H1299, H1975, A549, H460, GLC82, H460, and CALU-3 cell lines were provided by the American Type Culture Collection and maintained in RPMI-1640 supplemented with 10% FBS in a humidified atmosphere with 5% CO2. CALU-3 GEF-R is a cell line obtained in vitro as previously described. Briefly, over a period of 12 months, human CALU-3 lung adenocarcinoma cells were continuously exposed to increasing concentrations of gefitinib. The starting dose was the dose causing the inhibition of 50% of cancer cell growth (IC50; gefitinib, 1 μmol/L). The drug dose was progressively increased to 15 μmol/L in approximately 2 months, to 20 μmol/L after other 2 months, to 25 μmol/L after additional 2 months, and, finally, to 30 μmol/L for a total of 12 months. The established resistant cancer cell lines were then maintained in continuous culture with the maximally achieved dose of each TKI that allowed cellular proliferation (30 μmol/L for each drug) [2]. |
| Animal Research | Four- to 6-week old female balb/c athymic (nu+/nu+) mice were purchased from Charles River Laboratories. Mice were acclimatized for 1 week before being injected with cancer cells and injected subcutaneously with 107 H1299 and CALU-3 GEF-R cells that had been resuspended in 200 μL of Matrigel. When established tumors of approximately 75 mm3 in diameter were detected, mice were left untreated or treated with oral administrations of metformin (200 mg/mL metformin diluted in drinking water and present throughout the experiment), gefitinib (150 mg/kg daily orally by gavage), or both for the indicated time periods. Each treatment group consisted of 10 mice. Tumor volume was measured using the formula π/6 × larger diameter × (smaller diameter)2. Tumor tissues were collected from the xenografts and analyzed by Western blotting for the expression and activation of EGFR, AMPK, mitogen-activated protein kinase (MAPK), and S6 [2]. |
| In vitro | METHODS: Twenty-three tumor cells were treated with Gefitinib for 72 h, and cell viability was measured by MTT.
RESULTS: Only the PC9 cell line had an IC50 <1 μmol/L (highly sensitive), 14 cell lines had an IC50 >10 μmol/L (resistant), and the remaining 8 cell lines had an IC50 of 1-10 μmol/L (moderately sensitive). [1]
METHODS: Tumor cells HT29, KB, Du145 and A549 were treated with Gefitinib (0.032-50 μM) for 2 h. EGF (0.1 μg/mL) was added five minutes prior to cell lysis, and the expression levels of target proteins were detected using Western Blot.
RESULTS: Gefitinib produced a dose-dependent inhibition of EGFR autophosphorylation in all tumor cell lines. [2] |
| In vivo | METHODS: To detect anti-tumor activity in vivo, Gefitinib (3.125-200 mg/kg in 0.5% polysorbate 80) was administered orally to nude mice harboring tumors A431, Du145, or A549 once a day for seven to fifteen days.
RESULTS: Gefitinib inhibited the growth of A431, Du145 or A549 tumors in a dose-dependent manner. [2]
METHODS: To assay antitumor activity in vivo, Gefitinib (40 mg/kg once daily) or Gefitinib (200 mg/kg every five days) was administered by gavage for two weeks to athymic nude mice harboring the human lung cancer tumor H3255.
RESULTS: Weekly treatment showed better inhibition than daily treatment. Compared with the daily dosing regimen, the weekly dosing regimen showed stronger inhibition of p-EGFR, p-ERK, and p-AKT. [3] |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 18.33 mg/mL (41.02 mM)
Ethanol : 4.5 mg/mL (10 mM)
10% DMSO+40% PEG300+5% Tween 80+45% Saline : 4.47 mg/mL (10 mM), Please add co-solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately.
|
| Keywords | EGFR tyrosine kinase | Autophagy | lung cancer | breast cancer | phosphorylation | Inhibitor | Apoptosis | inhibit | TAMs | NSCLCs | EGFR | antitumour | Epidermal growth factor receptor | HER1 | tumor metastasis | Gefitinib | ZD-1839 | ZD 1839 | ErbB-1 |
| Related Compound Libraries | Bioactive Compound Library | Membrane Protein-targeted Compound Library | Inhibitor Library | Natural Product Library for HTS | FDA-Approved Drug Library | Anti-Cancer Drug Library | Anti-Cancer Active Compound Library |

 United States
United States