Factory Provide Hydroxycitric Acid Powder Hca 60% CAS 6205-14-7 Garcinia Cambogia Extract

Introduction
Product Name : Garcinia Cambogia Extract /Hydroxycitric acid
Other name:Rattan yellow fruit extract;Garcinia cambogia extract;TRIPOTASSIUM-2-HYDROXYCITRATE;Tripotassium-2-Hydroxycditracte;HAC;HYDROXYCITRICACIDANDITSTRISODIUMSALT;
CAS No.: 6205-14-7
MF:C6H8O8
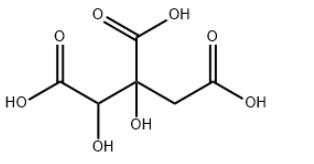
MW:208.12
Specification:99%
Appearance: White to off white powder
Melting Point :393.3±42.0 °C(Predicted)
Density :1.947±0.06 g/cm3(Predicted)
Storage :Keep in dark place,Inert atmosphere,Room temperature
| Prduct Name | Garcinia Cambogia Extract | Active ingredient | Hydroxy Citric Acid |
| Specification | 60% | Test Method | HPLC |
| Appearance | Off-white Fine Powder | CAS No. | 90045-23-1 |
| Particle Size | 98% pass 80mesh | Storage | Cool & dry place |
Garcinia cambogia ( it was also called tamarind fruit) is a tropical fruit grown in Indonesia. It contains rich calcium, phosphorus, iron, thiamine, riboflavin, and niacin. The active ingredients of its rind hydroxycitric is Hydroxycitric Acid (HCA) which decreases appetite and prevents your body from storing food as fat. But garcinia cambogia extract diet pills are supplements, not drugs. Its medicinal effectiveness is always controversial.
Garcinia cambogia extract is non-toxic, tasteless, odorless powder and found to be very effective herbal alternate for controlling obesity and cholesterol by inhibiting lipogenesis in our body. Garcinia Cambogia has been used for thousands of years in the Orient as a food supplement. It is used as an appetite suppressant and to inhibit the absorption and synthesis of fat, cholesterol and triglycerides.
| ANALYSIS ITEMS | SPECIFICATION | TEST METHOD |
| Appearance | Fine powder | Organoleptic |
| Color | Off white | Visual |
| Odour & Taste | Characteristic | Organoleptic |
| Identification | Identical to R.S. sample | HPTLC |
| Hydroxy Citric Acid | 50.0%,60.0% | HPLC |
| Solubility | Not soluble in water |
|
| Hydrocarbons PAHs | ≤ 50 ppb -Reg.UE 1933/2015 | GC-MS |
| Benzo(a)pyren | ≤ 10 ppb -Reg.UE 1933/2015 | GC-MS |
| Radioactivity | ≤ 600 Bq/Kg -Reg. CE 1409/2009 |
|
| Apparent density | 0.4-0.7g/ml |
|
| Sieve Analysis | 100% through 80 mesh | USP39 <786> |
| Loss on drying | ≤ 5.0% | Eur.Ph.9.0 [2.5.12] |
| Total Ash | ≤ 5.0% | Eur.Ph.9.0 [2.4.16] |
| Lead (Pb) | ≤ 3.0 mg/kg | Eur.Ph.9.0<2.2.58>ICP-MS |
| Arsenic (As) | ≤ 1.0 mg/kg | Eur.Ph.9.0<2.2.58>ICP-MS |
| Cadmium(Cd) | ≤ 1.0 mg/kg | Eur.Ph.9.0<2.2.58>ICP-MS |
| Mercury(Hg) | ≤ 0.1 mg/kg -Reg.EC629/2008 | Eur.Ph.9.0<2.2.58>ICP-MS |
| Heavy metal | ≤ 10.0 mg/kg | Eur.Ph.9.0 <2.4.8> |
| Solvents Residue | Conform Eur.ph. 9.0 <5,4 > and EC European Directive 2009/32 | Eur.Ph.9.0<2.4.24> |
| Pesticides Residues | Conform Regulations(EC) No.396/2005 including annexes and successive updates Reg.2008/839/CE | Gas Chromatography |
| Aerobic bacteria(TAMC) | ≤1000 cfu/g | USP39 <61> |
| Yeast/Moulds(TAMC) | ≤100 cfu/g | USP39 <61> |
| Bile-tol.gram- b./Enterobact.: | ≤100 cfu/g |
|
| Escherichia coli: | Absent in 1g | USP39 <62> |
| Salmonella spp: | Absent in 25g | USP39 <62> |
| Staphylococcus aureus: | Absent in 1g |
|
| Listeria Monocytogenens | Absent in 25g |
|
| Aflatoxins B1 | ≤ 5 ppb -Reg.EC 1881/2006 | USP39 <62> |
| Aflatoxins ∑ B1, B2, G1, G2 | ≤ 10 ppb -Reg.EC 1881/2006 | USP39 <62> |
Function
1. Inhibit the synthesis of fat and prevent the conversion of sugars into excess fat in the body.
2. It can regulate fat metabolism in the human body.
3. Increase the ability of liver and muscle to store glycogen.
4. Has the effect of suppressing appetite.
APPLICATIONS
1.Health care raw materials:
HCA can loss weight; suppress appetite, blocks the conversion of proteins and carbohydrates into fats and slows the formation of fatty acids and cholesterol.
2. Pharmaceutical raw materials:
HCA can change the body's lipid and carbohydrate metabolism, and has the effect of reducing blood lipids.







 China
China




