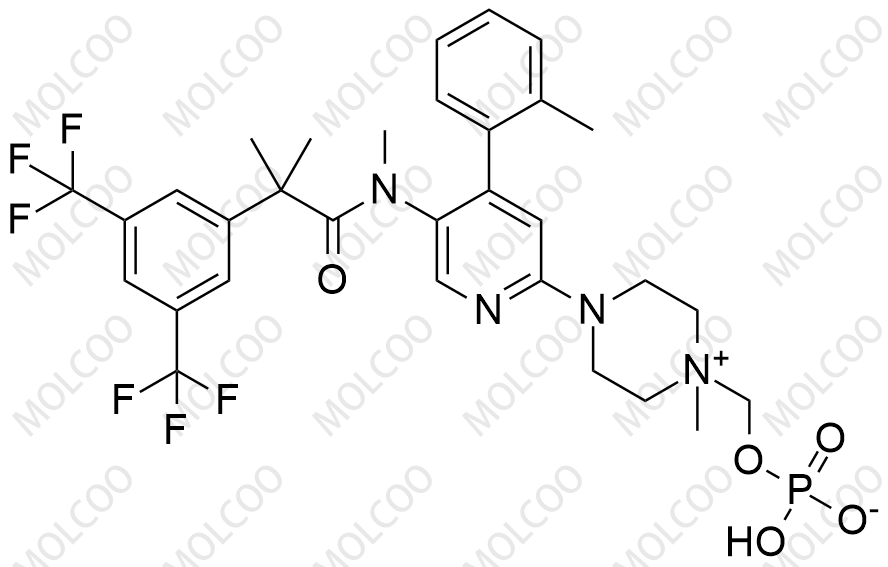
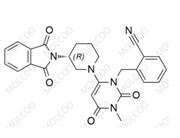
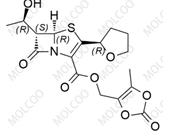
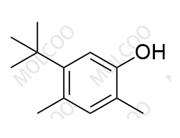
Fosnetupitant 1703748-89-3
Fosnetupitant Impurity Reference Standards – High-Purity Tools for Drug Development
Product Overview
Fosnetupitant (CAS: 1703748-89-3) is a novel NK1 receptor antagonist prodrug used in the prevention of chemotherapy-induced nausea and vomiting (CINV). To meet the needs of drug R&D, quality analysis, and bioequivalence studies, we offer high-purity Fosnetupitant impurity reference standards, empowering research institutions and pharmaceutical companies to accelerate project timelines.
Key Features
Purity Assurance: HPLC purity ≥98%, with select batches reaching 99%. COA reports and HNMR, MS, HPLC spectra provided.
Structural Verification: Comprehensive data including IR, UV, and 2D NMR (COSY, NOESY, HMBC, HMQC) for unambiguous impurity identification.
Rapid Supply: In-stock availability with flexible packaging (10mg–100g). Expedited shipping for urgent requests.
Regulatory Support: Impurity source analysis, QC strategy development, and analytical method optimization services.
Applications
Drug Discovery: Impurity structural elucidation, metabolite profiling, and degradation pathway studies.
Quality Control: Impurity limit determination, stability testing, and lot-to-lot consistency evaluation.
Bioequivalence: Impurity spectrum comparison for generic drug development.
Technical Expertise
Over a decade of experience in impurity R&D, with custom synthesis capabilities.
Advanced SFC purification technology for complex impurity isolation.
End-to-end solutions including process scale-up and CMC optimization.






 China
China