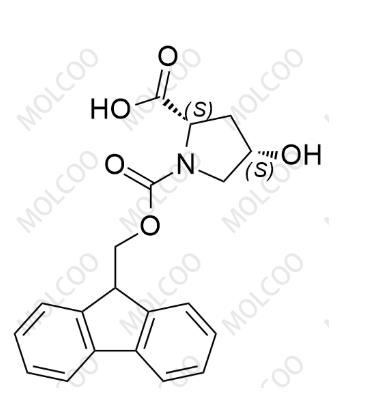
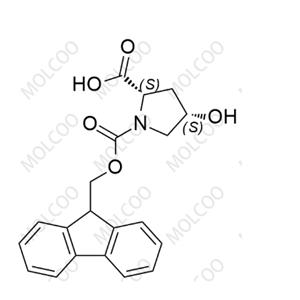
Fomc-cis-L-hydroxyproline-OH
Product Code:B201360
English Name:Fomc-cis-L-hydroxyproline-OH
English Alias:(2S,4S)-1-(((9H-fluoren-9-yl)methoxy)carbonyl)-4-hydroxypyrrolidine-2-carboxylic acid
CAS No.:189249-10-3
Molecular Formula:C₂₀H₁₉NO₅
Molecular Weight:353.37
High Purity:Confirmed by HPLC (≥99.0%), NMR (1H, 13C), HRMS, and elemental analysis, suitable for precise modification of cis-hydroxyproline derivatives in peptide synthesis.
Excellent Stability:Stable for 36 months at -20℃ under light-protected, sealed storage; well-soluble in common organic solvents (e.g., DMF, DCM) with degradation rate <0.5% within 6 months, ensuring synthesis reliability.
Peptide Drug Synthesis:As an Fmoc-protected cis-hydroxyproline monomer, used in solid-phase peptide synthesis (SPPS) to construct functional peptides containing hydroxyproline, such as antifibrotic peptides and enzyme inhibitors.
Drug Development Tool:Modulates peptide conformational stability and biological activity by introducing hydroxyproline structure in new drug development, e.g., improving serum stability or target binding capacity.
Biochemical Research:Used to study protein-protein interactions or as a probe molecule to label biomacromolecules, regulating molecular recognition properties via hydroxyl hydrophilicity.
Hydroxyproline, an important non-natural amino acid, widely exists in natural products like collagen, and its cis configuration is crucial for maintaining protein secondary structure. Fomc-cis-L-hydroxyproline-OH, as an Fmoc-protected cis-isomer, protects the amino group with fluorenylmethoxycarbonyl (Fmoc) to avoid racemization during synthesis while retaining the reactivity of the 4-hydroxy group. This compound enhances peptide structural rigidity in peptide drug design, often used to develop therapeutic peptides with specific spatial conformations, such as anti-tumor and anti-inflammatory drugs.
Synthesis Process:Prepared by condensation of fluorenylmethoxycarbonyl chloride with cis-L-hydroxyproline under alkaline conditions (e.g., NaHCO₃/DCM system), with optimized reaction temperature (0-5℃) and purification (reverse-phase preparative HPLC), yielding over 75%.
Application Progress:Recent studies show that peptides containing this monomer exhibit excellent activity in treating fibrotic diseases, such as reducing collagen deposition by inhibiting TGF-β signaling; additionally, used as a linker component in ADC drugs to modify antibodies or toxin molecules via hydroxyl groups, improving conjugate stability.
Analytical Techniques:Circular dichroism (CD) and X-ray crystallography verify β-turn structures induced in peptides, while UPLC-MS/MS monitors impurities (e.g., trans-isomers, deprotected products) during synthesis to ensure product uniformity.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China