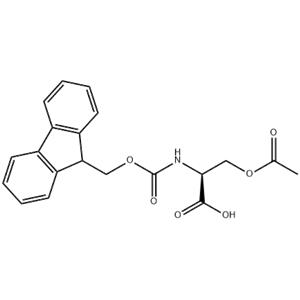
Solid-Phase Peptide Synthesis (SPPS):Fmoc-Ser(Ac)-OH is a valuable building block in solid-phase peptide synthesis. The presence of the Fmoc group allows for selective deprotection using base, enabling the coupling of this amino acid with other amino acids in a stepwise manner.
The acetyl group on the serine residue protects the hydroxyl group from unwanted reactions during the peptide synthesis process. This protection is removed at a later stage to reveal the free hydroxyl group, which can participate in additional reactions or serve as a functional group for further modifications. Peptide Libraries and Drug Discovery:Fmoc-Ser(Ac)-OH can be used in the synthesis of peptide libraries, where the acetyl group on the serine residue provides a handle for further modifications. These modifications can include the attachment of various ligands, probes, or drug candidates to the peptide backbone. These peptide libraries can be screened for binding affinity, biological activity, or selectivity against target molecules. This approach is useful in drug discovery, where peptides containing Fmoc-Ser(Ac)-OH may lead to the identification of novel therapeutic agents. Biological Assays and Protein-Ligand Interactions:Peptides containing Fmoc-Ser(Ac)-OH can be used in biological assays to study protein-ligand interactions. The acetyl group on the serine residue can be removed to expose the hydroxyl group, allowing for the formation of hydrogen bonds or other interactions with protein binding sites. These peptides can be labeled with fluorescent probes or other detection reagents to monitor binding events in vitro or in vivo. They can also be used to elucidate the binding mechanism or affinity of ligands for specific protein targets. | 
 China
China