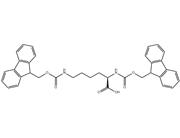
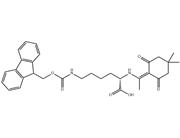
Fmoc-Lys(Ddiv)-OH is a synthetic amino acid derivative commonly used in peptide synthesis, particularly in solid-phase peptide synthesis (SPPS). It belongs to the class of Fmoc (fluorenylmethoxycarbonyl)-protected amino acids, which are widely employed in this technique due to their solubility and stability properties.
The specific application of Fmoc-Lys(Ddiv)-OH is as a building block for the synthesis of peptides containing lysine residues. Lysine is an essential amino acid in proteins and plays a crucial role in various biological functions, including protein cross-linking and binding to other molecules. By incorporating Fmoc-Lys(Ddiv)-OH into a peptide sequence, researchers can precisely control the position and number of lysine residues in the final peptide product.
The "Ddiv" moiety in Fmoc-Lys(Ddiv)-OH refers to a divinyl sulfone protecting group that is used to protect the epsilon-amino group of lysine during peptide synthesis. This protecting group is stable under the conditions of SPPS but can be removed under specific conditions, allowing for the subsequent modification or conjugation of the lysine residue with other molecules.
In the context of peptide synthesis, Fmoc-Lys(Ddiv)-OH allows for the preparation of peptides with lysine residues that can be further modified or functionalized. This is particularly useful in the development of peptide-based drugs, bioconjugates, or probes where lysine residues play a critical role in binding affinity, selectivity, or bioactivity. | 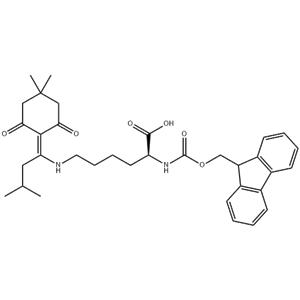

 China
China