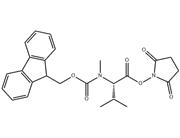
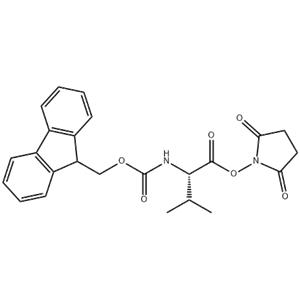
Synonyms: | CarbaMicacid,[1-[[(2,5-dioxo-1-pyrrolidinyl)oxy]carbonyl]-2-Methylpropyl]-,9H-fluoren-9-ylMethylester,(S)-;L-Valine,N-[(9H-fluoren-9-ylmethoxy)carbonyl]-,2,5-dioxo-1-pyrrolidinylester;N-Fmoc-L-valineN-succinimidylester;Fmoc-L-valineN-hydroxysuccinimideester≥99%(HPLC);FMOC-L-VALINEN-HYDROXYSUCCINIMIDEESTER;FMOC-VAL-OSU;FMOC-VALINE-OSU;NALPHA-9-Fluorenylmethoxycarbonyl-L-valineN-hydroxysuccinimideester |
Application: |
Building Block for Peptide Synthesis: In the process of peptide chain synthesis, Fmoc-L-Val-Osu serves as an activated form of L-valine, which can be linked with other amino acid residues to gradually build the target peptide. The Fmoc (9-fluorenylmethoxycarbonyl) protecting group stabilizes the amino acid during synthesis, while the Osu (succinimidyl ester) moiety facilitates the reaction with amino groups, promoting the formation of peptide bonds.
Protein Modification and Labeling: Through the introduction of Fmoc-L-Val-Osu or its derivatives, valine or related moieties can be incorporated into proteins for site-specific modification or labeling. This modification can alter the properties, functions, or activities of proteins, enabling studies on protein structure, function, or interactions.
Solid-Phase Peptide Synthesis: In solid-phase peptide synthesis, Fmoc-L-Val-Osu, as a soluble amino acid derivative, can react with amino acid residues on a solid-phase support, allowing for the stepwise elongation of the peptide chain. This method is suitable for synthesizing longer and more complex peptide sequences. Biochemical Research: The introduction of activated amino acids such as Fmoc-L-Val-Osu enables the study of amino acid metabolism, interactions, and recognition mechanisms with other molecules in biological systems. This is crucial for understanding fundamental biochemical processes in life. |

 China
China